Phase 2/3 study evaluating safety, immunogenicity, and noninferiority of single booster dose of AVX/COVID-12 vaccine
- PMID: 40577471
- PMCID: PMC12204155
- DOI: 10.1126/sciadv.adq2887
Phase 2/3 study evaluating safety, immunogenicity, and noninferiority of single booster dose of AVX/COVID-12 vaccine
Abstract
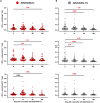
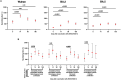
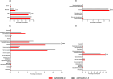
Low- and middle-income countries face substantial challenges in immunizing against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including high costs, limited access, and insufficient local manufacturing. To address these issues, we developed and locally manufactured the AVX/COVID-12 vaccine using a cost-effective Newcastle disease virus LaSota platform to express a stabilized SARS-CoV-2 spike protein (HexaPro-S). We evaluated the AVX/COVID-12 vaccine in a phase 2/3 parallel-group, double-blind, active-controlled, noninferiority trial with 4056 volunteers, demonstrating its safety, good tolerability, and ability to induce neutralizing antibodies against ancestral SARS-CoV-2 and the Omicron BA.2 and BA.5 variants. It also stimulated interferon-γ-producing CD8+ T cells and met the World Health Organization's noninferiority criteria compared to AZ/ChAdOx-1-S. No vaccinated participants experienced severe disease, hospitalization, or death. These findings support the use of AVX/COVID-12 as a booster to help achieve and maintain population immunity while addressing global inequities in vaccine distribution, and it has been approved for adult booster use in Mexico.
Figures


References
-
- WHO COVID-19 dashboard, https://covid19.who.int/.
-
- COVID-19 pandemic, https://ourworldindata.org/coronavirus.
-
- COVID19 vaccine tracker, https://covid19.trackvaccines.org/.
-
- Global dashboard for vaccine equity, 18 April 2021; https://data.undp.org/vaccine-equity/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

