Hepatic NMNAT1 is required to defend against alcohol-associated fatty liver disease
- PMID: 40577472
- PMCID: PMC12204165
- DOI: 10.1126/sciadv.adt6195
Hepatic NMNAT1 is required to defend against alcohol-associated fatty liver disease
Abstract
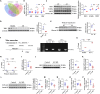
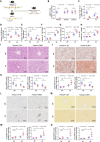
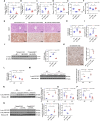
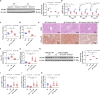
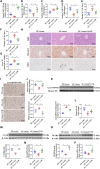
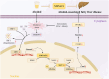
Nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1), a nicotinamide adenine dinucleotide (NAD+) synthetase in Preiss-Handler and salvage pathways, governs nuclear NAD+ homeostasis. This study investigated the role of NMNAT1 in alcohol-associated liver disease (ALD). Decreased NMNAT1 expression and activity were observed in the liver of patients with alcohol-associated hepatitis and either liver or primary hepatocytes from ALD mice. F-box and WD repeat domain containing 7 (FBXW7)-regulated interferon regulatory factor 1 (IRF1) ubiquitination degradation contributed to the alcohol-inhibited NMNAT1 transcriptional level. Hepatic NMNAT1 knockout aggravated alcohol-induced hepatic NAD+ decline and further hepatic steatosis and liver injury. Metabolomics and transcriptomics interaction revealed that the cysteine sulfinic acid decarboxylase (CSAD)-regulated taurine pathway was involved in NMNAT1-disrupted hepatic lipid metabolism in ALD. Hepatic CSAD overexpression or taurine supply attenuated hepatic NMNAT1 knockout-aggravated ALD. Hepatic NMNAT1 loss inhibited NMN-protected ALD. Replenishing hepatic NMNAT1 reversed liver lipid accumulation in ALD mice. These findings identified NMNAT1 as a promising therapeutic target for ALD.
Figures


References
-
- Seitz H. K., Bataller R., Cortez-Pinto H., Gao B., Gual A., Lackner C., Mathurin P., Mueller S., Szabo G., Tsukamoto H., Alcoholic liver disease. Nat. Rev. Dis. Primers. 4, 16 (2018). - PubMed
-
- French S. W., Chronic alcohol binging injures the liver and other organs by reducing NAD+ levels required for sirtuin’s deacetylase activity. Exp. Mol. Pathol. 100, 303–306 (2016). - PubMed
-
- Hao L., Sun Q., Zhong W., Zhang W., Sun X., Zhou Z., Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: A molecular mechanism of protection against alcoholic liver disease. Redox Biol. 14, 626–636 (2018). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

