Metachromatic Leukodystrophy: New Therapy Advancements and Emerging Research Directions
- PMID: 40577679
- PMCID: PMC12205745
- DOI: 10.1212/WNL.0000000000213817
Metachromatic Leukodystrophy: New Therapy Advancements and Emerging Research Directions
Abstract
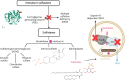
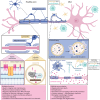
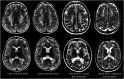
Metachromatic leukodystrophy (MLD) is a rare autosomal recessive lysosomal storage disorder caused by disease-causing variants in the gene coding for arylsulfatase A, leading to deficient enzyme activity and subsequent accumulation of sulfatides. MLD is characterized by demyelination and neurodegeneration of the central and peripheral nervous system, manifesting as progressive motor and cognitive defects in affected individuals. This review provides a comprehensive overview of the significant progress made in MLD research in the past decade, regarding natural history, disease and treatment mechanisms, and newborn screening (NBS). Traditionally, MLD has been classified according to age at onset (late-infantile, early-juvenile and late-juvenile, and adult MLD), with earlier forms leading to more rapid neurologic decline. New data show that the type of presenting symptoms further influences the dynamic of disease progression. Patients with a cognitive presentation have a much slower or even no motor decline than patients with a mixed motor and cognitive presentation. Research advancements have enabled improved understanding of the effects of allogeneic hematopoietic stem cell transplantation and the development of novel therapeutic approaches, including hematopoietic stem cell gene therapy, which is now authorized in the EU, United Kingdom, and United States as treatment for selected patients with early-onset forms of MLD. Both hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy are most effective when administered before disease onset. To identify presymptomatic patients, NBS for MLD is becoming available in several countries, resulting in new challenges. Decisions regarding patient eligibility for these treatments in already symptomatic individuals, as well as the timing of treatment for patients identified through NBS, require thorough understanding of disease progression. Biomarkers may be helpful for disease staging and prediction of disease evolution. Moreover, apart from timing, challenges remain regarding optimal treatment strategies across MLD subtypes, especially late-onset MLD, and management of the clinical heterogeneity and course of the disease. Another important issue is ensuring therapy accessibility, which forms a substantial barrier for equitable care. Continued research and international collaboration are essential to address these challenges, with the goal of improving care and outcomes for patients with MLD and their families.
Conflict of interest statement
M.A.B.C. Asbreuk, D.H. Schoenmakers, L.A. Adang, S. Beerepoot, C. Bergner, A. Bley, J.J. Boelens, V. Calbi, A. García-Cazorla, E.A. Eklund, F. Fumagalli, S.W. Grønborg, S. Groeschel, P.M. Van Hasselt, C.E.M. Hollak, S.A. Jones, T.J. de Koning, L. Laugwitz, C. Lindemans, F. Mochel, A. Øberg, D. Ram, L. Schöls, C. Sevin, A. Zerem, and N.I. Wolf are members of the MLD initiative, which is an academic registry and collaborative platform for metachromatic leukodystrophy. The MLD initiative was a case study at the program “Managing patient registries for expensive drugs” and received funding from the Dutch Healthcare Institute between April 2021 and March 2027. N.I. Wolf is an advisor and/or coinvestigator for premarketing clinical trials in metachromatic leukodystrophy and other leukodystrophies (Shire/Takeda, Orchard, Ionis, PassageBio, VigilNeuro, Sana Biotech, Lilly), without personal payment. M.A.B.C. Asbreuk, D.H. Schoenmakers, and C.E.M. Hollak are members of platform “Medicijn voor de Maatschappij,” an academic initiative to support sustainable access to medicines for rare diseases. This platform is financially supported by a grant from “de Nationale Postcode Loterij,” a National Lottery that distributes funds raised by this lottery for good causes primarily concerning health and welfare in Netherlands. L.A. Adang is a consultant and/or coinvestigator for clinical trials in metachromatic leukodystrophy and other leukodystrophies (Shire/Takeda, Orchard Therapeutics, Ionis, Lilly). A. Bley is a site subinvestigator for the Takeda SHP611 trial; and received travelling support from Orchard-Tx. J.J. Boelens received consulting fees from Sobi, Sanofi, SmartImmune, and Merck. V. Calbi and F. Fumagalli are investigators of haematopoietic stem cell gene therapy clinical trials for MLD sponsored by Orchard Therapeutics, the license holder of investigational medicinal product OTL-200. Both are ad hoc consultants for the advisory board of Orchard Therapeutics. A. García-Cazorla has participated in an advisory board organized by Orchard Therapeutics. E.A. Eklund participated in several advisory boards arranged by Orchard Therapeutics. S.W. Grønborg participated in an Orchard Therapeutics advisory board. S. Groeschel received institutional research grants from Shire (a Takeda company) and Orchard Therapeutics; and is an advisor for Clario and Orchard Therapeutics, without personal payments. C.E.M. Hollak is involved in pre-marketing studies with pharmaceutical companies (Sanofi, Protalix, Idorsia) without personal fees. S.A. Jones is an investigator and consultant for Orchard and Takeda. T.J. de Koning has received an unrestricted grant and speaker fee from PTC Pharmaceuticals, unrelated to MLD; and received a grant from the Dutch Brain Foundation (DR_2023-00428 on progressive myoclonus epilepsy). C. Lindemans is an ad hoc advisor in Orchard Therapeutics and Bluebird Bio expertise panels. F. Mochel participated in advisory boards arranged by Minoryx Therapeutics and Vigil Neuroscience; and her research work is funded by the French Ministry of Health (PHRC), the French Ministry of Research (ANR), the Paris Brain Institute, and Minoryx Therapeutics. D. Ram is a principal investigator of the Takeda clinical trial and consultant for Orchard Therapeutics. L. Schöls is consultant to Vico Therapeutics, Alexion, and Novartis; and is a site principal investigator for trials of Vigil Neuroscience, Stealth Biotherapeutics, and PTC Therapeutics, all unrelated to MLD. C. Sevin is an advisor and/or investigator for clinical trials in MLD and other leukodystrophies (Bluebird Bio, Shire/Takeda, Minoryx Therapeutics, Orchard, Ionis). A. Zerem is a coinvestigator for clinical trials in MLD and other leukodystrophies (Shire/Takeda, Ionis, Minoryx Therapeutics). F.M. Vaz is a consultant for Scenic Biotech. All other authors report no disclosures relevant to the manuscript. Go to
Figures

References
-
- Gomez-Ospina N. Arylsulfatase A deficiency. In: GeneReviews. University of Washington, Seattle; 2024.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
