The ecology and plasticity of fish skin and gill microbiomes: seeking what matters in health and disease
- PMID: 40577810
- PMCID: PMC12218203
- DOI: 10.1093/femsre/fuaf027
The ecology and plasticity of fish skin and gill microbiomes: seeking what matters in health and disease
Abstract
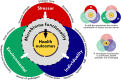
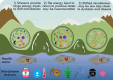
The microbiomes of skin and gill mucosal surfaces are critical components in fish health and homeostasis by competitively excluding pathogens, secreting beneficial compounds, and priming the immune system. Disruption of these microbiomes can compromise their capacity for disease resilience and maintaining host homeostasis. However, the extent and nature of microbiome disruption required to impact fish health negatively remains poorly understood. This review examines how various stressors influence the community composition and functionality of fish skin and gill microbiomes, and the subsequent effects on fish health. Our findings highlight that the impact of stressors on skin and gill microbiomes may differ for different body sites and are highly context-dependent, influenced by a complex interplay of host-specific factors, stressor characteristics, and environmental conditions. By evaluating current knowledge on the genesis and homeostasis of these microbiomes, we highlight a strong influence of environmental factors especially on skin and gill microbiomes compared with fish gut microbiomes, which appear to be more closely regulated by the host's homeostatic and immunological systems. This review emphasizes the importance of understanding the ecology and plasticity of fish skin and gill microbiomes to identify critical thresholds for microbiome shifts that impact fish health and disease resilience.
Keywords: animal health; aquaculture; dysbiosis; environment; immune; mucous; stressor.
© The Author(s) 2025. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Unveiling the Characteristics of Microbiota in Different Mucosal Layers of Leopard Coral Grouper (Plectropomus leopardus).Mar Biotechnol (NY). 2025 May 20;27(3):86. doi: 10.1007/s10126-025-10458-5. Mar Biotechnol (NY). 2025. PMID: 40392419
-
Stronger together: harnessing natural algal communities as potential probiotics for inhibition of aquaculture pathogens.Microbiol Spectr. 2025 Jul;13(7):e0042125. doi: 10.1128/spectrum.00421-25. Epub 2025 May 21. Microbiol Spectr. 2025. PMID: 40396728 Free PMC article.
-
Temperature sensitivity of the interspecific interaction strength of coastal marine fish communities.Elife. 2023 Jul 11;12:RP85795. doi: 10.7554/eLife.85795. Elife. 2023. PMID: 37431235 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Akazawa N, Alvial A, Blanc P-P et al. Reducing disease risk in aquaculture. World Bank Report Number 88257-GLB. Washington, DC: World Bank, 2014. 10.13140/RG.2.1.4525.5529. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

