Longitudinal profiling of hormone receptor positive, HER2 negative metastatic breast cancer through droplet digital PCR-based circulating tumor DNA fragmentomics
- PMID: 40577963
- PMCID: PMC12268100
- DOI: 10.1016/j.tranon.2025.102456
Longitudinal profiling of hormone receptor positive, HER2 negative metastatic breast cancer through droplet digital PCR-based circulating tumor DNA fragmentomics
Abstract
Background: In the context of hormone receptor positive, HER2 negative Metastatic breast cancer (MBC), CDK 4/6 inhibitors (CDK4/6i) combined with endocrine therapy represent the standard first-line treatment, improving Progression-Free Survival (PFS) and Overall Survival (OS). Despite these benefits, resistance to treatment develops, necessitating early risk classification to guide clinical management. This study explores the potential of cell-free DNA (cfDNA) fragmentomics, specifically ACTB fragments, in predicting tumor dynamics and treatment outcomes in luminal MBC, based on the principle that shorter DNA fragments are generally indicative of circulating tumor DNA (ctDNA) from tumor cells, while longer fragments are associated with leukocyte lysis.
Methods: In the MAGNETIC.1 study, 141 women with luminal-like MBC were enrolled between January 2018 and January 2023. Blood samples were collected at baseline (BL), and after 3 (T3) and 6 (T6) months of treatment. cfDNA was extracted and analyzed using droplet digital PCR (ddPCR) to quantify ACTB fragments (136 bp, 420 bp, and 2,000 bp). Continuous variables were compared using the Mann-Whitney test and Kruskall Wallis test depending on data distribution and number of groups. Categorical variables were compared using the Chi-square test or Fischer's exact test whenever appropriate. Differences in survival were tested by log-rank test and uni- and multivariable Cox regression.
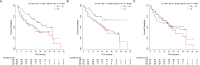
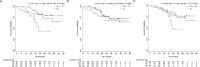
Results: By categorizing the values of actinic fragments into interquartiles (Q1, Q2, and Q3), ACTBshort Q3 at baseline was significantly associated with negative PR expression (RRR 0.27, P = 0.012) and a higher frequency of liver metastasis (RRR = 3.75, P = 0.009). In terms of clinical outcomes, regarding PFS a significant role was observed for baseline ACTBshort Q3 (HR 1.92, P = 0.041) and ACTBmedium Q3 (HR 0.47, P = 0.043), the latter maintaining significance in multivariable analysis (HR 0.33, 95 %, P = 0.012). For OS, ACTBshort Q3 demonstrated a significant impact in both univariable (HR 3.94, P = 0.003) and multivariable analyses (HR 3.25, P = 0.023).
Conclusions: This study demonstrates the feasibility of employing a fragmentomics mutation agnostic approach in luminal MBC. Baseline and longitudinal changes in ACTB fragments were significantly associated with clinical outcomes, suggesting their potential as non-invasive biomarkers for early risk classification and monitoring tumor dynamics.
Keywords: Circulating tumor DNA; Fragmentomics; Liquid biopsy; Metastatic breast cancer.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.B. reports advisory/consultancy fee from AstraZeneca, Lilly, MSD, Novartis, Pfizer, SeaGen; travel grants from Lilly, Roche. A.M. reports advisory/consultancy fee from Novartis, MSD, BMS, Merk, Sunpharma, PierreFabre, Gilead, Seagen, Genomic Health; invited speech from Novartis, MSD, BMS, Merk, Sunpharma, Sanophi, PierreFabre, AstraZeneca, Daiichi Sankyo; travel grants from Gilead, PierreFabre; other familial: MSD, AstraZeneca, Pharmamar, GSK. L.G. reports advisory/consultancy fee from AstraZeneca, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Incyte, Novartis, Pfizer, Merck Sharp & Dohme, Menarini Stemline, Abbvie; research funding from Menarini Silicon Biosystems. F.P. reports honoraria for advisory boards, activities as a speaker, travel grants, research grants from Amgen, Astrazeneca, Daiichi Sankyo, Celgene, Eisai, Eli Lilly, Exact Sciences, Gilead, Ipsen, Italfarmaco, Menarini, MSD, Novartis,Pierre Fabre, Pfizer, Roche, Seagen, Takeda, Viatris; Research funding from Astrazeneca – Eisai – Roche
Figures

References
-
- Migliaccio I., Bonechi M., McCartney A., Guarducci C., Benelli M., Biganzoli L., et al. CDK4/6 inhibitors: A focus on biomarkers of response and post-treatment therapeutic strategies in hormone receptor-positive HER2-negative breast cancer. Cancer Treat. Rev. 2021;93 doi: 10.1016/J.CTRV.2020.102136. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

