Exercise-induced mitochondrial protection in skeletal muscle of ovariectomized mice: A myogenic E2 synthesis-independent mechanism
- PMID: 40578025
- PMCID: PMC12266561
- DOI: 10.1016/j.redox.2025.103735
Exercise-induced mitochondrial protection in skeletal muscle of ovariectomized mice: A myogenic E2 synthesis-independent mechanism
Abstract
Background: Skeletal muscle, a 17β-estradiol (E2)-sensitive tissue, is prone to accelerated aging due to postmenopausal E2 deficiency and subsequent mitochondrial dysfunction. While exogenous E2 treatment has been shown to protect against mitochondrial damage in ovariectomized rodents, the impact of exercise-induced local E2 production in skeletal muscle on mitochondrial function remains to be determined. This study investigated exercise-mediated mitochondrial protection in ovariectomized mice and the contribution of myogenic E2.
Methods: Female C57BL/6J mice (8-week-old) were divided into Sham, OVX, and OVX + ET groups (N = 12). OVX mice underwent bilateral ovariectomy, with the OVX + ET group performing 8 weeks of treadmill exercise starting 10 weeks post-surgery. Functional tests (grip strength, fatigue resistance) and gastrocnemius analyses (morphology, mitochondrial function, E2/antioxidant levels, and protein expression) were conducted. Parallel experiments in muscle-specific aromatase knockout (MS-ARO-CKO) mice included E2 supplementation via subdermal pellets.
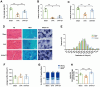
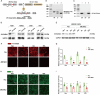
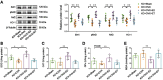
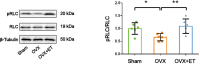
Results: 18 weeks after ovariectomy (OVX), C57BL/6J mice exhibited significant reductions in grip strength (∼30 %), rotarod performance (∼57 %), and grid hanging performance (∼92 %). Concomitantly, OVX led to marked decreases in mitochondrial respiration (p < 0.05) and antioxidant capacity (p < 0.05) in the gastrocnemius muscle, accompanied by alterations in mitochondrial quality control and antioxidant signaling proteins (p < 0.05). Exercise intervention effectively attenuated these OVX-induced deficits, accompanied by a 66 % increase in E2 levels and upregulation of aromatase (ARO) activity and expression (p < 0.05). In MS-ARO-CKO mice model, exercise failed to improve the impaired antioxidant capacity induced by OVX. However, exercise, similar to estrogen supplementation, restored mitochondrial function and related protein expression abnormalities induced by OVX (p < 0.05).
Conclusions: Our findings demonstrate that the protective effects of exercise on skeletal muscle mitochondria involve multiple mechanisms, independent myogenic E2 Synthesis, providing novel insights for improving skeletal muscle health in postmenopausal women.
Keywords: Exercise training; Mitochondrial function; Muscle weakness; Myogenic 17β-estradiol.
Copyright © 2025 Shanghai University of Sport , School of Exercise and Health. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest in relation to the manuscript titled "Exercise-Induced Mitochondrial Protection in Skeletal Muscle of Ovariectomized Mice: A Myogenic E(2) Synthesis-Independent Mechanism" submitted to Redox Biology. We confirm that the results and interpretations reported in the manuscript are original and have not been plagiarized.
Figures





Similar articles
-
APOE4 genotype negates the benefits of 17β-estradiol on cerebrovascular endothelial and mitochondrial function.bioRxiv [Preprint]. 2025 Jul 27:2025.07.23.666474. doi: 10.1101/2025.07.23.666474. bioRxiv. 2025. PMID: 40777460 Free PMC article. Preprint.
-
Genistein Enhances the Beneficial Effects of Exercise on Antioxidant and Anti-inflammatory Balance and Cardiomyopathy in Ovariectomized Diabetic Rats.Antiinflamm Antiallergy Agents Med Chem. 2025;24(2):103-113. doi: 10.2174/0118715230305886240916105248. Antiinflamm Antiallergy Agents Med Chem. 2025. PMID: 39482917
-
Aldehyde Oxidase 1 Deficiency Enhances Aerobic Exercise Performance by Promoting Skeletal Muscle Adaptation and Improving Mitochondrial Function.FASEB J. 2025 Jul 31;39(14):e70815. doi: 10.1096/fj.202500240R. FASEB J. 2025. PMID: 40673877 Free PMC article.
-
Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression.Sports Med. 2025 Jan;55(1):115-144. doi: 10.1007/s40279-024-02120-2. Epub 2024 Oct 10. Sports Med. 2025. PMID: 39390310 Free PMC article.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
References
-
- World Health Organization Falls. 2021. https://www.who.int/newsroom/fact-sheets/detail/falls
-
- Enns D.L., Tiidus P.M. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58. - PubMed
-
- Sipilä S., Taaffe D.R., Cheng S., Puolakka J., Toivanen J., Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101(2):147–157. - PubMed
-
- Ronkainen P.H., Kovanen V., Alén M., Pöllänen E., Palonen E.M., Ankarberg-Lindgren C., Hämäläinen E., Turpeinen U., Kujala U.M., Puolakka J., Kaprio J., Sipilä S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J. Appl. Physiol. 2009;107(1):25–33. 1985. - PubMed
-
- Phillips S.K., Rook K.M., Siddle N.C., Bruce S.A., Woledge R.C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84(1):95–98. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

