How meristems shape plant architecture in cereals-Cereal Stem Cell Systems (CSCS) Consortium
- PMID: 40578304
- PMCID: PMC12290889
- DOI: 10.1093/plcell/koaf150
How meristems shape plant architecture in cereals-Cereal Stem Cell Systems (CSCS) Consortium
Abstract
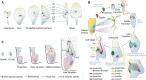
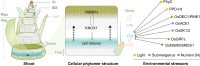
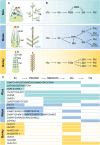
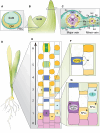
Meristems are major determinants of plant architecture, diversification, and acclimation to environmental stresses. Moreover, meristems play also a major role during crop domestication and are fundamentally important for the productivity of crop plants as they directly determine biomass and grain yield. While vegetative meristems shape the basic plant body plan and produce all above- and below-ground parts of plants, some vegetative meristems transit to reproductive meristems, forming sexual organs and germ cells. Most knowledge about plant meristems was generated using the model plant Arabidopsis. Compared with Arabidopsis, architecture of grass or cereals, including crops like maize, wheat, barley, rice and sorghum, is more complex: cereals produce additional organs like a coleoptile, seminal roots originating from the scutellar nodes in the embryo and shoot-borne crown roots as well as highly complex inflorescence meristems with meristem types absent in eudicots. Moreover, studies in cereals indicated that paradigms based on studies using Arabidopsis are not universally applicable. This review therefore aims to provide a comprehensive overview about the initiation, establishment, maintenance, and function of the various cereal meristems and their stem cell niches that shape our most important crop plants. Stem cell-like systems involved in leaf pattering and germline formation are also considered. The focus is also on the significant progress that has been made recently using novel tools to elucidate gene regulatory networks underlying the development and function of the various cereal meristems.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Regulating Plant Architecture to Enhance the Future of Cereal Crop Production.Physiol Plant. 2025 Jul-Aug;177(4):e70367. doi: 10.1111/ppl.70367. Physiol Plant. 2025. PMID: 40579794 Review.
-
Fabricating mice and dementia: opening up relations in multi-species research.In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. PMID: 40690569 Free Books & Documents. Review.
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355

