Global, regional, and national trends in routine childhood vaccination coverage from 1980 to 2023 with forecasts to 2030: a systematic analysis for the Global Burden of Disease Study 2023
- PMID: 40578370
- PMCID: PMC12338332
- DOI: 10.1016/S0140-6736(25)01037-2
Global, regional, and national trends in routine childhood vaccination coverage from 1980 to 2023 with forecasts to 2030: a systematic analysis for the Global Burden of Disease Study 2023
Abstract
Background: Since its inception in 1974, the Essential Programme on Immunization (EPI) has achieved remarkable success, averting the deaths of an estimated 154 million children worldwide through routine childhood vaccination. However, more recent decades have seen persistent coverage inequities and stagnating progress, which have been further amplified by the COVID-19 pandemic. In 2019, WHO set ambitious goals for improving vaccine coverage globally through the Immunization Agenda 2030 (IA2030). Now halfway through the decade, understanding past and recent coverage trends can help inform and reorient strategies for approaching these aims in the next 5 years.
Methods: Based on the Global Burden of Diseases, Injuries, and Risk Factors Study 2023, this study provides updated global, regional, and national estimates of routine childhood vaccine coverage from 1980 to 2023 for 204 countries and territories for 11 vaccine-dose combinations recommended by WHO for all children globally. Employing advanced modelling techniques, this analysis accounts for data biases and heterogeneity and integrates new methodologies to model vaccine scale-up and COVID-19 pandemic-related disruptions. To contextualise historic coverage trends and gains still needed to achieve the IA2030 coverage targets, we supplement these results with several secondary analyses: (1) we assess the effect of the COVID-19 pandemic on vaccine coverage; (2) we forecast coverage of select life-course vaccines up to 2030; and (3) we analyse progress needed to reduce the number of zero-dose children by half between 2023 and 2030.
Findings: Overall, global coverage for the original EPI vaccines against diphtheria, tetanus, and pertussis (first dose [DTP1] and third dose [DTP3]), measles (MCV1), polio (Pol3), and tuberculosis (BCG) nearly doubled from 1980 to 2023. However, this long-term trend masks recent challenges. Coverage gains slowed between 2010 and 2019 in many countries and territories, including declines in 21 of 36 high-income countries and territories for at least one of these vaccine doses (excluding BCG, which has been removed from routine immunisation schedules in some countries and territories). The COVID-19 pandemic exacerbated these challenges, with global rates for these vaccines declining sharply since 2020, and still not returning to pre-COVID-19 pandemic levels as of 2023. Coverage for newer vaccines developed and introduced in more recent years, such as immunisations against pneumococcal disease (PCV3) and rotavirus (complete series; RotaC) and a second dose of the measles vaccine (MCV2), saw continued increases globally during the COVID-19 pandemic due to ongoing introductions and scale-ups, but at slower rates than expected in the absence of the pandemic. Forecasts to 2030 for DTP3, PCV3, and MCV2 suggest that only DTP3 would reach the IA2030 target of 90% global coverage, and only under an optimistic scenario. The number of zero-dose children, proxied as children younger than 1 year who do not receive DTP1, decreased by 74·9% (95% uncertainty interval 72·1-77·3) globally between 1980 and 2019, with most of those declines reached during the 1980s and the 2000s. After 2019, counts of zero-dose children rose to a COVID 19-era peak of 18·6 million (17·6-20·0) in 2021. Most zero-dose children remain concentrated in conflict-affected regions and those with various constraints on resources available to put towards vaccination services, particularly sub-Saharan Africa. As of 2023, more than 50% of the 15·7 million (14·6-17·0) global zero-dose children resided in just eight countries (Nigeria, India, Democratic Republic of the Congo, Ethiopia, Somalia, Sudan, Indonesia, and Brazil), emphasising persistent inequities.
Interpretation: Our estimates of current vaccine coverage and forecasts to 2030 suggest that achieving IA2030 targets, such as halving zero-dose children compared with 2019 levels and reaching 90% global coverage for life-course vaccines DTP3, PCV3, and MCV2, will require accelerated progress. Substantial increases in coverage are necessary in many countries and territories, with those in sub-Saharan Africa and south Asia facing the greatest challenges. Recent declines will need to be reversed to restore previous coverage levels in Latin America and the Caribbean, especially for DTP1, DTP3, and Pol3. These findings underscore the crucial need for targeted, equitable immunisation strategies. Strengthening primary health-care systems, addressing vaccine misinformation and hesitancy, and adapting to local contexts are essential to advancing coverage. COVID-19 pandemic recovery efforts, such as WHO's Big Catch-Up, as well as efforts to bolster routine services must prioritise reaching marginalised populations and target subnational geographies to regain lost ground and achieve global immunisation goals.
Funding: The Bill & Melinda Gates Foundation and Gavi, the Vaccine Alliance.
Copyright © 2025 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests R A Abeldano Zuniga reports consulting fees from the UN International Organization for Migration; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the University of Helsinki; outside the submitted work. Q E Adnani reports grants or contracts from the Online Library Data Research funds from Universitas Padjadjaran, Bandung, Indonesia, under contract number 2152/UN6.3.1/PT.00/2024 and Scientific Excellence Research Grant from Universitas Padjadjaran, Bandung, Indonesia, under contract number 908/UN6.3.1/PT.00/2025; outside the submitted work. S Afzal reports grants or contracts from Intellectual contributions and time protection was given by Dean office Institute of Public Health Lahore; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by the Dean Institute of Public Health Lahore; Support for attending meetings and/or travel from the Dean Institute of Public Health Lahore, Pakistan; participation on a data safety monitoring board or advisory board as a Member Pakistan National Bioethics Committee, Member Institutional Review Board of Fatima Jinnah Medical University, Member Ethical Review Board and Data Monitoring Board Institute of Public Health Lahore Pakistan, In charge Clinical Research Organization King Edward Medical University; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Member Pakistan Higher Education Commission Research Committee, Member Pakistan Medical and Dental Commission Research and Journals Committee, Member Pakistan Society of Internal Medicine, Member Pakistan Association of Medical Editors, Member Medical Microbiology and Infectious Diseases Society, Fellow of Leads International, Fellow of Faculty of Public Health UK, Fellow of College of Physicians and Surgeons Pakistan; Receipt of equipment from Bergen University Norway for research writing; Other financial or non-financial support from Dean Public Health and Preventive Medicine King Edward Medical University; outside the submitted work. A Aguilera reports grants or contracts from Pfizer Biopharmaceutical Group Andean Cluster (Chile) employee; outside the submitted work. A Amin reports the following pending patents: US20200253891A1; Method of Liver Cancer Treatment with Safranal-Based Formulations; US20200254049A1; Combination Therapy for Cancer; US20200253890A1; Suppression and Inhibition of CDC25B with Safranal-Based Formulations, outside the submitted work. A Bhagavathula reports support for attending meetings and/or travel from the American College of Epidemiology, North Dakota State University, GE Health Care, and NIG to attend meetings and travel in 2024-2025; Leadership or fiduciary role in other board, society, committee or advocacy group, unpaid as a member of the Board of Directors of the American College of Epidemiology, Associate Editor of Jmir Public Health and Surveillance, Associate Editor of Frontiers in Public Health – Digital Health Section, and Associate Editor af Frontiers in Cardiovascular Medicine; outside the submitted work. S Bhaskar reports grants or contracts from Japan Society for the Promotion of Science (JSPS), Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Grant-in-Aid for Scientific Research (KAKENHI; Grant ID: 23KF0126), JSPS and the Australian Academy of Science, JSPS International Fellowship (Grant ID: P23712; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as District Chair, Diversity, Equity, Inclusion & Belonging of Rotary District 9675 (Sydney, Australia), as Chair, Founding Member and Manager of the Global Health & Migration Hub Community, Global Health Hub Germany (Berlin, Germany), as Editorial Board Member of PLOS One, BMC Neurology, Frontiers in Neurology, Frontiers in Stroke, Frontiers in Public Health, Journal of Aging Research, Neurology International, Diagnostics, & BMC Medical Research Methodology, as a member of the College of Reviewers, Canadian Institutes of Health Research (CIHR), Government of Canada, as the Director of Research of World Headache Society (Bengaluru, India), as Expert Adviser/Reviewer of Cariplo Foundation (Milan, Italy), as Visiting Director of National Cerebral and Cardiovascular Center, Department of Neurology, Division of Cerebrovascular Medicine and Neurology, Suita (Osaka, Japan), as Member, Scientific Review Committee of Cardiff University Biobank (Cardiff, UK), as Chair of Rotary Reconciliation Action Plan, and Healthcare and Medical Adviser at Japan Connect (Osaka, Japan); outside the submitted work. D-T Chu reports grants or contracts from the Vietnam National Foundation for Science and Technology Development (NAFOSTED; the Vietnam National University, Hanoi (VNU) VNU International School (as a PI unless otherwise indicated) by the Vietnam National University Hanoi (VNU) for the 2025 research activities of the VNU strong re-search group - the interdisciplinary research group on biomedicine and health (IRGBH; The establishing Decision no. 4013/QĐ-ĐHQGHN on October 24, 2023), by the Vietnam National Foundation for Science and Technology Development (NAFOSTED (grant number 106.02-2019.314), by the Vietnam National University, Hanoi (VNU) under project ‘Researching on some clinical, non-clinical, and epidemiological characteristics, and genetic mutation and expression in Vietnamese patients with ovarian cancer’ (decision number 776/QĐ-ĐHQGHN), and other internal research grants from VNU International School from 2021 to now; outside the submitted work. J Condo reports grants or contracts from OncoNanoAI: Artificial intelligence to discover the next generation of personalized nanoparticles for triple-negative breast cancer therapy (2025-2027) FCT Grant LISBOA2030-FEDER-00862500-14998; Patents planned, issued or pending from TRPV2 Antagonists. U.S. Application No. US11273152B2,Surfactant-based cellulose hydrogel methods and uses thereof, PCT/IB2025/051694, 17/02/2025, Self-immolative micelle, methods and uses thereof, EP25165757, 24/03/2025; outside the submitted work. M Del Riccio reports grants or contracts from Moderna; Support for attending meetings and/or travel from MSD, Sanofi, and Astrazeneca; outside the submitted work. D Dias da Silva reports grants or contracts from Laboratório Associado para a Química Verde-Tecnologias e Processos Limpos (FCT/MECI, Fundação para a Ciência e Tecnologia and Ministério da Educação, Ciência e Inovação) through the project UID/50006-Laboratório Associado para a Química Verde–Tecnologias e Processos Limpos; outside the submitted work. X Ding reports grants or contracts from the American Heart Association (2-year predoctoral fellowship (DOI: 10.58275/AHA.25PRE1373497.pc.gr.227106); quarterly payments made to my institution), outside the submitted work. L M Ebraheim reports support for the present manuscript from the Gates Foundation and Gavi; and royalties or licenses from the Institute for Health Metrics and Evaluation. A Faro reports support for the present manuscript from National Council for Scientific and Technological Development (CNPq, Brazil; Personal grant “Researcher on Productivity at CNPq - Level 2”). N Fullman reports grants or contracts from the Gates Foundation; Other financial or non-financial interests from Gates Ventures (June 2020 to present), outside the submitted work. N Ghith reports grants or contracts from the Technical University of Denmark between 2019-2022 was covered by a grant from Novo Nordisk Foundation (NNF16OC0021856); Support for attending meetings and/or travel from Danish Data Science Institute at the Technical University of Denmark, travel grant in 2023; outside the submitted work. E Haeuser reports support for the present manuscript from the Gates Foundation and Gavi (joint grant #INV-037425 with the Gates Foundation and Gavi, with payments made to the institution). I Ilic reports support for the present manuscript from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (no. 451-03-137/2025-03/200110). M Ilic reports support for the present manuscript from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (no. 451-03-47/2023-01/200111). J Jozwiak reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, ADAMed, and AMGen; outside the submitted work. K Krishan reports other non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University (Chandigarh, India), outside the submitted work. J Liu reports support for the present manuscript from the National Natural Science Foundation (72474005) and Beijing Natural Science Foundation (L222027). E Lytvyak reports grants or contracts from the College of Physicians and Surgeons of Alberta, the Government of Alberta, and Advanz Pharma; Other interests as an employee of the University of Alberta and appointment of Alberta Health Services; outside the submitted work. S Y Ma'aruf reports leadership or fiduciary roles in other board, society, committee, or advocacy group, paid or unpaid as a volunteer Director of Research and Development at InnovateHealth Africa (IHA); outside the submitted work. H R Marateb repots grants or contracts from 2024 LLAV 00083, AGAUR (Agency for Management of University and Research Grants) and UPC Universitat Politècnica de Catalunya; outside the submitted work. M Marks-Hulstram reports grants or contracts from the Swedish Heart-Lung Foundation and Regional Research Foundation Middle Sweden (paid to institution); Royalties or licenses from Liber (intensivvård); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the Swedish Association for Anesthesiology and Intensive Care; Support for attending meetings and/or travel from American Physiological Society and the European Society of Intensive Care Medicine; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from the American Physiological Society; outside the submitted work. S Masi reports consulting fees from Servier; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier; Support for attending meetings and/or travel from Servier; participation on a data safety monitoring board or advisory board from Servier; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from the Italian Society of Internal Medicine; outside the submitted work. R J Maude reports support for the present manuscript from Wellcome Trust [Grant number 220211] as it pro-vides core funding for Mahidol Oxford Tropical Medicine Research and contributes to my salary. I am required by Wellcome to acknowledge this grant in all publications. A-F Mentis reports leadership or fiduciary role in other board, society, committee or advocacy group paid or unpaid as an Editorial Board Member for “Systematic Reviews” journal, for “Annals of Epidemiology” journal, and as Associate Editor for “Translational Psychiatry”; outside the submitted work. S A Meo reports grants or contracts from Researchers Supporting Project, King Saud Universi-ty, Riyadh, Saudi Arabia (RSP-2025 R47); outside the submitted work. L Monasta reports support for the present manuscript from the Italian Ministry of Health (Ricerca Corrente 34/2017), payments made to the Institute for Maternal and Child Health IRCCS Burlo Garofolo. J F Mosser reports support for the present manuscript from the Gates Foundation and Gavi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Providence Regional Medical Center (Honorarium for continuing medical education presentation); Support for attending meetings and travel support from the Gates Foundation. S Nomura reports grant support for the present manuscript from Ministry of Education, Culture, Sports, Science and Technology of Japan (24H00663), Precursory Research for Embryonic Science and Technology from the Japan Science and Technology Agency (JPMJPR22R8). B Oancea reports support for the present manuscript from MRID, project PNRR-I8 no 842027778., contract no 760096. A Ortiz reports grants or contracts from Sanofi (IIS-FJD UAM), and grants to Universidad Autonoma de Madrid (UAM) as Director of the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes; Consulting fees from Astellas, Astrazeneca, Bioporto, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Lilly, Chiesi, Otsuka, Novo-Nordisk, Sysmex; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astellas, Astrazeneca, Bioporto, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Sobi, Menarini, Lilly, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and Spafarma; Support for attending meetings and/or travel from Astellas, Astrazeneca, Fresenius Medical Care, Boehringer-Ingelheim, Bayer, Sanofi-Genzyme, Chiesi, Sobi, Bayer; participation on a data safety monitoring board or advisory board from Astellas, Astrazeneca, Boehringer-Ingelheim, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Chiesi, Otsuka, Novo Nordisk, Sysmex; Leadership or fiduciary role in other board, society, committee or advocacy group, unpaid from Council ERA. SOMANE; outside the submitted work. S K Panda reports support for the present manuscript from Siksha ‘O’ Anusandhan (Deemed to be University); Grants or contracts from DST-GOVT. OF ODISHA (Letter No. 3444/ST). R Passera reports participation on a data safety monitoring board or advisory board from dello studio “Consolidation with ADCT-402 (loncastuximab tesirine) after immunochemotherapy: a phase II study in BTKi-treated/ineligible Relapse/Refractory Mantle Cell Lymphoma (MCL) patients” - FIL, Fondazione Italiana Linfomi, Alessandria (Italy); Leadership or fiduciary role in other board, society, committee or advocacy group, unpaid as a Member of the EBMT Statistical Committee, European Society for Blood and Marrow Transplantation, Paris (F) and a Past member 2020-2023 (biostatistician) of the IRB/IEC Comitato Etico AO SS. Antonio e Biagio Alessandria-ASL AL-VC (Italy); outside the submitted work. V C F Pepito reports grants or contracts from Sanofi Consumer Healthcare; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Zuellig Family Foundation; outside the submitted work. X Qi reports consulting fees from The City University of New York and University of Texas at Austin; outside the submitted work. L F Reyes reports grants or contracts from GSK, MSD, Pfizer; Consulting fees from GSK, MSD, Pfizer; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GSK, MSD, Pfizer; Support for attending meetings and/or travel from GSK; outside the submitted work. Y L Samodra reports grants or contracts from Taipei Medical University, Taiwan and NSTC – NTU, Taiwan; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Co-founder of Benang Merah Research Center, Indonesia; Other financial or non-financial interests from idebeasiswa.com as a Mentor; outside the submitted work. F Shahkarami reports grants or contracts from Tehran University of Medical Sciences (TUMS; Department of Internal Medicine); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from TUMS; Other financial or non-financial interests as a part-time physician (general practitioner) and health consultant; all outside the submitted work. V Sharma reports other financial or non-financial interests from DFSS (MHA)'s research project (DFSS28(1)2019/EMR/6) at Institute of Forensic Science & Criminology, Panjab University, Chandigarh, India, outside the submitted work. L M L R D Silva reports grants or contracts from SPRINT, Sport Physical Activity and Health Research & Innovation Center, Polytechnic of Guarda, 6300-559 Guarda, Portugal; RISE-Health, Faculty of Health Sciences, University of Beira Interior, 6201-506 Covilhã, Portugal; outside the submitted work. J A Singh reports consulting fees from ROMTech, Atheneum, Clearview healthcare partners, American College of Rheumatology, Yale, Hulio, Horizon Pharmaceuticals, DINO-RA, ANI/Exeltis, USA Inc., Frictionless Solutions, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the speaker's bureau of Simply Speaking; Support for attending meetings and/or travel from Simply Speaking; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as a past steering committee member of the OMERACT, an international organization that develops measures for clinical trials and receives arm's length funding from 12 pharmaceutical companies, as Chair of the Veterans Affairs Rheumatology Field Advisory Committee, and as Editor and Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; Stock or stock options in Atai life sciences, Kintara therapeutics, Intelligent Biosolutions, Acumen pharmaceutical, TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp., Aebona Pharmaceuticals, and Charlotte's Web Holdings, Inc. and previously owned stock options in Amarin, Viking and Moderna pharmaceuticals; outside the submitted work. R Tabares-Seisdedos reports grants or contracts from the Valencian Regional Government's Ministry of Education (PROME-TEO/CIPROM/2022/58 and the Spanish Ministry of Science, Innovation and Universities (PID2021-129099OB-I00; The funders were not involved in the design of the manuscript or decision to submit the manuscript for publication, nor will they be involved in any aspect of the study's conduct); outside the submitted work. T Tabuchi reports grants or contracts from Daiichi Sankyo Healthcare, Data Seed Inc., Johnson & Johnson K.K., Workout-Plus; outside the submitted work. J H V Ticoalu reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as the co-founder of Benang Merah Research Center, Indonesia; outside the submitted work. S Tromans reports grants or contracts from part of the 2023/4 Adult Psychiatric Morbidity Survey team, collecting epidemiological data on community-based adults living in England (this is a contracted study from NHS Digital, via the Department of Health and Social Care), multiple chapters of the 2023/4 Adult Psychiatric Morbidity Survey report, Co-lead on a study from Jazz Pharmaceuticals related to reviewing the impact of medicinal cannabis on patients with the epilepsy syndromes Lennox-Gastaut syndrome and Dravet syndrome, lead on a study from the National Institute for Health and Care Research related to reviewing a national training programme for health and social care professionals relating to learning disability and autism, co-applicant on studies funded by the National Institute for Health and Care Research related to (1) medicine support interventions and strategies for people with learning disabilities and (2) Identification, recording, and reasonable adjustments for people with a learning disability and autistic people in NHS electronic clinical record systems, co-lead on a study investigating multiple antipsychotic prescribing in adults under the care of specialist learning disability services (funded by the Baily Thomas Charitable Fund); all payments made to institutions. Support for attending meetings and/or travel from the Royal College of Psychiatrists for accommodation and travel to conference events due to my academic secretary role in the faculty of the Psychiatry of Intellectual Disability and conference fees for Royal College of Psychiatrists events; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Academic Secretary for the Neurodevelopmental Psychiatry Special Interest Group and Psychiatry of Intellectual Disability Faculty at the Royal College of Psychiatrists; Editorial Board Member for Progress in Neurology and Psychiatry, Advances in Mental Health and Intellectual Disability, Advances in Autism, BMC Psychiatry, and BJPsych Open. Editor of Psychiatry of Intellectual Disability Across Cultures (Oxford University Press), outside the submitted role. E Upadhyay reports patents planned, issued or pending; A system and method of reusable filters for anti-pollution mask, A system and method for electricity generation through crop stubble by using microbial fuel cells, A system for disposed personal protection equipment (PPE) into biofuel through pyrolysis and method, A novel herbal pharmaceutical aid for formulation of gel and method thereof, Herbal drug formulation for treating lung tissue degenerated by particulate matter exposure, a method to transform cow dung into the wall paint by using natural materials and composition thereof, Biodegradable packaging composition and method of preparation thereof, Eco-friendly bio-shoe polish from banana and turmeric, Honey-based polyherbal syrup composition to treat air pollution-induced inflammation and preparation method thereof, Process for preparing a caffeine free, antioxidant and nutrient rich beverage; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Executive Council Member, Indian Meteorological Society, Jaipur Chapter (India) and as Member Secretary-DSTPURSE Program; outside the submitted work. J Ward reports support for the present manuscript from NIHR Clinical Lecturer 2022 – 2025. P Willeit reports consulting fees from Novartis Pharmaceuticals; outside the submitted work. J Wu reports grants or contracts from the National Heart, Lung, and Blood Institute (R38HL167238) and acknowledges prior funding from the American Society of Hematology, Hematology Opportunities for the Next Generation of Research Scientists (HONORS) Award; outside the submitted work. Y Yasufuku reports grants or contracts from Shionogi & Co; outside the submitted work. G Zamagni reports support for the present manuscript from the Italian Ministry of Health (Ricerca Corrente 34/2017), payments made to the Institute for Maternal and Child Health IRCCS Burlo Garofolo. A B Zemariam reports support for the present manuscript from funding provided by the Gates Foundation and Gavi. The funding agency has no role in data collection and interpretation.
Figures
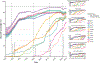



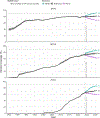
References
-
- World Health Assembly 27. WHO Expanded Programme on Immunization. 1974. https://iris.who.int/handle/10665/92778 (accessed Oct 22, 2024).
-
- World Health Organization. Essential Programme on Immunization. https://www.who.int/teams/immunization-vaccines-and-biologicals/essentia... (accessed July 30, 2024).
-
- Institute for Health Metrics and Evaluation (IHME). Financing Global Health 2023: The Future of Health Financing in the Post-Pandemic Era. Seattle, WA: IHME, 2024. https://www.healthdata.org/sites/default/files/2024-05/FGH_2023_Accessib....
-
- Sim SY, Watts E, Constenla D, Brenzel L, Patenaude BN. Return on investment from immunization against 10 pathogens in 94 low- and middle-income countries, 2011–30. Health Aff (Millwood) 2020; 39: 1343–53. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

