Physicochemical Comparison of Kolliphor HS 15, ELP, and Conventional Surfactants for Antibody Stabilization in Biopharmaceutical Formulations
- PMID: 40578813
- PMCID: PMC12326363
- DOI: 10.1021/acs.molpharmaceut.5c00519
Physicochemical Comparison of Kolliphor HS 15, ELP, and Conventional Surfactants for Antibody Stabilization in Biopharmaceutical Formulations
Abstract
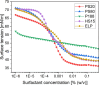
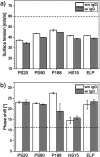
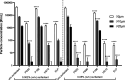
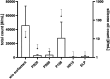
Stabilization of therapeutic protein formulations with nonionic surfactants such as Polysorbate 20 (PS20), Polysorbate 80 (PS80), or Poloxamer 188 (P188) is imperative to avoid critical loss of the active pharmaceutical ingredient by aggregation or adsorption onto different types of interfaces. In the present work, we have characterized the interfacial activity of the aforementioned surfactants, alone and in competition with antibodies, in comparison to two other excipients with approval for use in parenteral applications, Kolliphor HS 15 (HS15) and Kolliphor ELP (ELP). To this end, we applied a comprehensive suite of experimental techniques, including tensiometry, interfacial rheology, and quartz-crystal microbalance with dissipation monitoring (QCM-D). The obtained data shows important differences between the surfactants as well as a clear influence of the type of interface considered on the observed behavior. In order to link these physicochemical results to the performance of the chosen surfactants in the stabilization of antibodies, we performed another series of tests to quantify protein aggregation (i.e., the formation of (sub)visible particles in formulations under stress) as well as the release of oil from siliconized vials. In addition, the stability of the surfactants against enzymatic degradation was investigated. It is demonstrated that HS15 can compete with the widely used polysorbates in terms of interfacial activity and protein stabilization, while offering higher robustness against degradation by a lipase and an esterase. On the other hand, P188 shows poor interfacial activity but can still suppress the aggregation of at least some proteins, indicating that different mechanisms of stabilization are at play. Our findings and the broad spectrum of tests described in this work are instructive toward a better understanding of protein stabilization in distinct primary packaging systems through surfactants in aqueous formulations.
Keywords: antibodies; container closure; quartz-crystal microbalance; surface activity; surfactants.
Figures




Similar articles
-
Competitive Adsorption of Monoclonal Antibodies and Nonionic Surfactants at the Air-Water Interface.ACS Appl Mater Interfaces. 2025 Jul 16;17(28):40116-40128. doi: 10.1021/acsami.5c07162. Epub 2025 Jul 1. ACS Appl Mater Interfaces. 2025. PMID: 40590319
-
Monoacyl phospholipids to replace polysorbates as interfacial stabilizers in parenteral monoclonal antibody formulations.Eur J Pharm Sci. 2025 Sep 1;212:107191. doi: 10.1016/j.ejps.2025.107191. Epub 2025 Jul 3. Eur J Pharm Sci. 2025. PMID: 40617264
-
Comparison of Protein Particle Formation in IgG1 mAbs Formulated with PS20 Vs. PS80 When Subjected to Interfacial Dilatational Stress.AAPS PharmSciTech. 2023 Apr 20;24(5):104. doi: 10.1208/s12249-023-02561-4. AAPS PharmSciTech. 2023. PMID: 37081185 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Shire, S. J. Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product; Woodhead Publishing, 2015.
-
- BASF Pharma Technical Information - Kolliphor PS 20, November 2022, Available: https://pharma.basf.com/products/kolliphor-ps-20 (accessed 2025–06–11)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

