Effects of intravaginal conjugated oestrogen on pessary continuation for pelvic organ prolapse: multicentre, randomised, double blind, placebo controlled trial
- PMID: 40578851
- PMCID: PMC12310159
- DOI: 10.1136/bmj-2025-084418
Effects of intravaginal conjugated oestrogen on pessary continuation for pelvic organ prolapse: multicentre, randomised, double blind, placebo controlled trial
Abstract
Objective: To examine the effect of vaginal oestrogen cream on pessary continuation rates in pelvic organ prolapse.
Design: Multicentre, randomised, double blind, placebo controlled trial.
Setting: 12 academic medical centres in China (May 2020-June 2023).
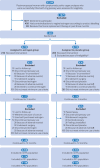
Participants: Postmenopausal women with symptomatic pelvic organ prolapse ≥stage 2 and successfully fitted with ring pessaries were randomly assigned in a 1:1 ratio to receive oestrogen cream or placebo cream.
Interventions: One gram of conjugated oestrogen cream (0.625 mg/g) or placebo cream was inserted vaginally every night for the first two weeks after successful pessary fitting followed by twice weekly for 12 months.
Main outcome measures: The primary outcome was the pessary continuation rate with satisfaction, which was defined as the proportion of participants who continued using the pessary and reported a response of very much better or much better on the Patient Global Impression of Improvement questionnaire at 12 months. Secondary outcomes included self-reported pelvic floor symptoms and adverse events. All analyses were based on a modified intention-to-treat approach, including participants who had at least one visit.
Results: Of 420 postmenopausal women randomised, 411 had at least one visit and were included in the modified intention-to-treat analysis (208 in the vaginal oestrogen group and 203 in the placebo group). The mean age of participants was 66 years. Pessary continuation rate with satisfaction did not differ significantly between the oestrogen group and the placebo group (181/208 (87.0%) v 176/203 (86.7%); risk difference 0.3%, 95% confidence interval -6.2% to 6.9%; P=0.92). Excessive discharge (34/208 (16.3%) v 52/203 (25.6%); -9.3%, -17.1% to -1.4%), vaginal erosion or ulcer (4/208 (1.9%) v 14/203 (6.9%); -5.0%, -8.9% to -1.0%), and vaginal bleeding (3/208 (1.4%) v 13/203 (6.4%); -5.0%, -8.7% to -1.2%) were less common in the vaginal oestrogen group.
Conclusions: Oestrogen cream did not improve the continuation rate of ring pessary use with satisfaction. Use of oestrogen cream might be associated with a lower risk of common adverse events. The clinical decision to use vaginal oestrogen should take into account its benefits and risks and the patient's personal preferences.
Trial registration: ClinicalTrials.gov NCT04393194.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: LZ received grants from the National Key R&D Program of China (2023YFC2706000); YinZ received grants from the National Key R&D Program of China (2023YFC2706001) and National High-level Hospital Clinical Research Funding (No 2022-PUMCH-C-031); non-financial support was from Xinjiang Nuziline Bio-Pharmaceutical Co for the oestrogen and placebo cream during the conduct of the study; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
