Selective neoadjuvant therapy of rectal cancer patients (SELREC): study protocol for a European randomised controlled, open, multicentre non-inferiority trial
- PMID: 40578870
- PMCID: PMC12207177
- DOI: 10.1136/bmjopen-2024-092807
Selective neoadjuvant therapy of rectal cancer patients (SELREC): study protocol for a European randomised controlled, open, multicentre non-inferiority trial
Abstract
Introduction: Neoadjuvant (chemo)radiotherapy (n(C)RT) followed by resection with total mesorectal excision (TME) constitutes the standard treatment for patients with locally advanced rectal cancer of the middle and lower third. However, n(C)RT has demonstrated no significant impact on overall survival but is associated with adverse effects, including impaired sphincter and sexual function. We hypothesise that omitting n(C)RT in selected patients with a clear circumferential resection margin (CRM) >1 mm as determined through preoperative MRI is not inferior regarding local recurrence rate within 3 years after surgery. That treatment approach may show fewer adverse effects and be more cost-effective.
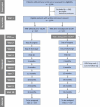
Methods and analysis: Selective neoadjuvant therapy of rectal cancer patients (SELREC) is a randomised controlled, parallel-group, open, multicentre, non-inferiority trial. The experimental intervention involves performing TME surgery without n(C)RT. In contrast, the control intervention adheres to German S3-guidelines, incorporating neoadjuvant radiotherapy (nRT) with a dosage of 5×5 Gy or a total of 50.4 Gy. Additionally, if applicable, concomitant chemotherapy (CT) based on 5-fluorouracil is administered, followed by TME surgery within less than 12 weeks. Adjuvant treatment according to guidelines is allowed depending on the (y)pTNM stage.The inclusion criteria for this study encompass adult patients with primary adenocarcinoma of the rectum in whom the main tumour mass is located less than 12 cm away from the anal verge, as assessed via proctoscopy. Additionally, eligible participants are required to have a preoperative tumour stage determined by MRI of either T1 or T2 with lymph node involvement (N1) or T3 with no lymph node involvement (N0) or with lymph node involvement (N1) and no distant metastases (M0). The assessment of a clear CRM >1 mm, based on MRI, is another prerequisite for inclusion. A total of 1074 patients in approximately 35 centres are planned to be allocated to the trial.The primary endpoint of the trial is local recurrence within 3 years after surgery. The primary estimand is based on the full analysis set using a logistic mixed model (margin 3%). The first secondary endpoint is no/minor low anterior resection syndrome (LARS) score at 2 years after surgery, and further secondary endpoints include survival outcomes and quality of life. Safety analysis involves describing the frequencies of major intervention-specific complications, such as the acute toxicity of n(C)RT according to CTCAE and perioperative morbidity and mortality according to Clavien-Dindo criteria.SELREC is financially supported by the German Federal Ministry of Education and Research.
Ethics and dissemination: This trial has been prospectively registered in the German Clinical Trials Register.Previously, the study had been approved by the responsible ethics committee of Heidelberg and the local ethics committees of the collaborating institutions before patient enrolment. Any protocol deviation that has an impact on relevant parameters such as study design, endpoints or patient safety will be reported to the responsible ethics committees.The results will be published in a peer-reviewed scientific journal and on institutional websites.
Trial registration number: German Clinical Trials Register DRKS00030567.
Keywords: Colorectal surgery; Magnetic resonance imaging; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. doi: 10.1016/S0140-6736(09)60484-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources