Randomised controlled trial to assess the impact of hospital-community pharmaceutical care on drug-related problems in oncology practice for at-risk outpatients treated with oral anticancer drugs-a French Society for Oncology Pharmacy (SFPO) study: DROP-SFPO study protocol
- PMID: 40578885
- PMCID: PMC12207170
- DOI: 10.1136/bmjopen-2024-094825
Randomised controlled trial to assess the impact of hospital-community pharmaceutical care on drug-related problems in oncology practice for at-risk outpatients treated with oral anticancer drugs-a French Society for Oncology Pharmacy (SFPO) study: DROP-SFPO study protocol
Abstract
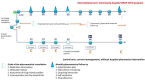
Introduction: Drug-related problems (DRPs) associated with oral anticancer drugs are frequent and require a new healthcare organisation to manage them on an outpatient basis. The aim of this article is to present the study protocol of the Drug Related problems in Oncology Practice (DROP) randomised controlled trial (RCT), endorsed by the French Society for Oncology Pharmacy. The main objective of the DROP RCT is to measure the impact at 6 months of the DROP community/hospital pharmaceutical intervention programme, compared with usual treatment, on the mean number of DRP (ie, adverse effects, drug-drug interactions, medication errors) related to oral anticancer drugs in at-risk outpatients.
Methods and analysis: The DROP protocol is a prospective, multicentre controlled clinical trial, with individual randomisation, comparing in parallel and in open, two groups of outpatients treated with oral anticancer drugs. The interventional group benefits from the DROP multidisciplinary intervention on oral anticancer treatment. The control group receives usual care. The primary outcome of the DROP RCT is the number of DRP due to oral anticancer drugs, per patient, identified between the inclusion of the patient and 6 months after inclusion ETHICS AND DISSEMINATION: Approval to conduct this study was obtained for all participating centres from an Ethics Committee (Comité de Protection des Personnes Sud-Méditerranée V) in August 2018 in accordance with French law. The trial results will be disseminated at clinical conferences and published in peer-reviewed journals.
Trial registration number: ClinicalTrials.gov: NCT03257969, recruitment started in June 2019. The current protocol version is V.9, 13 December 2023.
Keywords: CHEMOTHERAPY; Drug Utilization; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Timely post-discharge medication reviews to Improve Continuity-the Transitions Of Care stewardship (TIC TOC) study in rural and regional Australia: a parallel-group randomised controlled trial study protocol.BMJ Open. 2025 Jun 18;15(6):e100588. doi: 10.1136/bmjopen-2025-100588. BMJ Open. 2025. PMID: 40533208 Free PMC article.
-
Professional, structural and organisational interventions in primary care for reducing medication errors.Cochrane Database Syst Rev. 2017 Oct 4;10(10):CD003942. doi: 10.1002/14651858.CD003942.pub3. Cochrane Database Syst Rev. 2017. PMID: 28977687 Free PMC article.
-
FERN: is it possible to conduct a randomised controlled trial of intervention or expectant management for early-onset selective fetal growth restriction in monochorionic twin pregnancy - protocol for a prospective multicentre mixed-methods feasibility study.BMJ Open. 2024 Aug 17;14(8):e080021. doi: 10.1136/bmjopen-2023-080021. BMJ Open. 2024. PMID: 39153765 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical