The real-world effectiveness of defocus incorporated multiple segments and highly aspherical lenslets on myopia control: a longitudinal study from the French myopia cohort
- PMID: 40578905
- PMCID: PMC12207109
- DOI: 10.1136/bmjophth-2025-002142
The real-world effectiveness of defocus incorporated multiple segments and highly aspherical lenslets on myopia control: a longitudinal study from the French myopia cohort
Abstract
Aims: To evaluate the efficacy of myopia control by spectacle lenses in a real-world study.
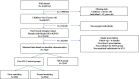
Methods: This is a longitudinal, retrospective, comparative, observational, real-world study of the French Myopia Cohort. Records of prescriptions for optical correction, gender and age were collected from 1500 opticians between 2020 and 2023. The study cohort consisted of myopic children aged 4 to 15 years who were assigned to three groups: two control groups wearing single vision lenses (SVL) and one intervention group wearing myopia control spectacles (MCS); either defocus incorporated multiple segments (DIMS, n=1786) or highly aspheric lenses (HAL, n=585). The first SVL group was matched to the MCS group for age, sex and initial refractive error (first matching), and the second SVL group was matched for the same criteria and myopia progression during the first 6 months of follow-up (second matching).The difference in myopia progression was calculated between SVL groups and the MCS group. DIMS and HAL were also compared for myopia progression.
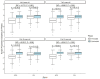
Results: A total of 2542 children (mean age of 9.5 years and mean spherical equivalent of -2.3 D at baseline) were included in each of the three groups. The mean progression rates for MCS were by +0.59 D (95% CI +0.57 to +0.62D; p<0.001) after the first matching and by +0.30 D (95% CI +0.28 to +0.32D; p<0.001) after the second matching, in comparison to the SVL groups. Children wearing HAL spectacles showed slightly less myopia progression (difference in progression = +0.14 D, 95% CI = +0.10 to +0.18, p<0.001) compared with the DIMS group.
Conclusions: Although there are some limitations, including its retrospective design, the lack of lifestyle and environmental data and the use of SE rather than axial length, this study showed that in a real-world setting, both DIMS and HAL spectacles demonstrated efficacy in reducing myopia progression. While a statistically significant lower myopia progression rate was observed in the HAL group, this difference was not clinically meaningful. This study also showed that DIMS and HAL reduce myopia progression among younger children aged 4 to 6 years.
Keywords: Child health (paediatrics); Optics and Refraction; Treatment Medical; Vision.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AMB has no financial disclosures. NL reports personal fees from Novartis, personal fees from Abbvie, personal fees from Bayer, from Essilor, from Hoya and from Santen, outside the submitted work. RN reports grants from Krys Group during the conduct of the study. MB reports personal fees from Alcon Research, inc., personal fees from Bruno Vision, inc., personal fees from Conexiant, personal fees from CooperVision, inc., personal fees from Dopavision, inc., personal fees from EssilorLuxottica, inc., personal fees from Euclid Systems, inc., personal fees from Eyenovia, inc., personal fees from Genentech, personal fees from Johnson & Johnson Vision, inc., personal fees from Kubota Vision, inc., personal fees from MJH Life Sciences, personal fees from NASEM, personal fees from Novartis, personal fees from Santen, inc., personal fees from Sydnexis, inc., personal fees from Thea, inc. and personal fees from Vyluma, outside the submitted work. MN reports grants from Siemens Healthineers outside the submitted work and from Krys Goup during the conduct of the study; CG reports grants from Siemens Healthineers outside the submitted work and Krys Group during the conduct of the study; and SR reports personal fees from Hoya, personal fees from Johnson & Johnson Vision, inc., personal fees from Vyluma and personal fees from Théa. RB has no financial disclosures. JBJ report European patent EP 3 271 392, JP 2021-119187 and U.S. Patent No. US 12,024,557 B2: 'Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia; European patent application 23196899.1'. EGFR Antagonists for the treatment of diseases involving unwanted migration, proliferation and metaplasia of retinal pigment epithelium (RPE) cells.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
