PLLA Coating of Lyophilized Human Bone Allograft for Long-term Release of Antibiotics
- PMID: 40578975
- PMCID: PMC12223631
- DOI: 10.21873/invivo.13987
PLLA Coating of Lyophilized Human Bone Allograft for Long-term Release of Antibiotics
Abstract
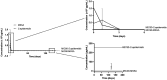
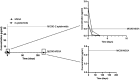
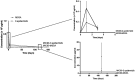
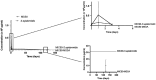
Background/aim: Mineralized allogeneic bone substitutes are ideal biomaterials for bone regeneration in surgeries. Moreover, they are also suitable for releasing antibiotics, delivering initial concentrations many times higher than the minimal inhibitory concentration for relevant bacterial strains, without delaying bone formation. In the present study, the potential of sustained-release long-term antibiotic delivery using allografts was investigated. A broad spectrum of antibiotics, including gentamicin, vancomycin, rifampicin, and clindamycin, was incorporated into the bone blocks using a poly-L-lactic acid (PLLA) polymer coating.
Materials and methods: An in-vivo model of implantation within the rabbit tibia plateau was used to track the sustained release of single/combined antibiotics for up to 120 days. Bony integration and tissue responses to the PLLA-coated antibiotic-delivery systems were analyzed at 4 months post implantation using histopathological analysis.
Results: The variant loaded with both vancomycin and rifampicin demonstrated the highest activity against methicillin-sensitive Staphylococcus aureus and Staphylococcus epidermidis. Histopathological analysis revealed that the tissue responses to the coated allogeneic bone substitutes were comparable in all study groups, with no observable histopathological differences. The coated bone blocks induced a strong foreign-body reaction, including high numbers of multinucleated giant cells and other immune cells but no material-associated bone growth.
Conclusion: Based on these results, future optimization can focus on selecting more efficient release of antibiotics and increasing the encapsulated concentration to sustain antibiotic release over 4 months, thereby improving the bacteriostatic effect in vivo. Furthermore, biocompatibility and osteoconductivity must be improved.
Keywords: Biocompatibility; MIC90; allograft; antibiotic susceptibility; bone graft infection; bone regeneration; drug delivery; local antibiotic delivery; rabbit tibia.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures

References
-
- Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier ML, Nazhat SN, Bouchard P, Chaussain C, Rochefort GY. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814. doi: 10.1038/srep38814. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
