Identifying the Primary Tumor Site and Distinguishing False-positives in Patients With Elevated Serum Carcinoembryonic Antigen
- PMID: 40579007
- PMCID: PMC12223601
- DOI: 10.21873/invivo.14035
Identifying the Primary Tumor Site and Distinguishing False-positives in Patients With Elevated Serum Carcinoembryonic Antigen
Abstract
Background/aim: Carcinoembryonic antigen (CEA) is a tumor marker that is frequently evaluated clinically for gastrointestinal and lung cancers. CEA is a glycoprotein antigen, and only the "value" measured by enzyme-linked immunosorbent assay is provided in the clinical setting. At present, no method has been established to indicate whether the value is a false-positive elevation or whether there is a primary cancer site. To obtain clues on how to identify the originating site in patients with cancer with high CEA levels and to identify CEA false-positives in healthy individuals, we conducted an exploratory study.
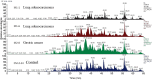
Patients and methods: A pilot study was performed using the multivariate analysis method and principal component analysis-discriminant analysis on proteomic results obtained using liquid chromatography-mass spectrometry (LC/MS) in two patients with lung cancer, one patient with gastric cancer, and one healthy control individual.
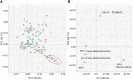
Results: No differences in specific proteins associated with high CEA levels were detected between lung and gastric cancers using LC/MS. Therefore, we performed statistical analysis using principal component analysis-discriminant analysis to determine whether there were differences in the protein signal patterns obtained using LC/MS. The results showed that the plots obtained for each patient and the healthy control were located in different quadrants of the four-quadrant matrix scatter plot.
Conclusion: Our results suggest the possibility of visually differentiating the primary tumor site in patients with elevated CEA levels. This method may also help recognize false-positive CEA results.
Keywords: CEA; Tumor marker; carcinoembryonic antigen; differential diagnosis.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to declare in relation to this study.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
The Diagnostic and Prognostic Value of the Combination of Tumor M2-Pyruvate Kinase, Carcinoembryonic Antigen, and Cytokeratin 19 Fragment in Non-Small Cell Lung Cancer.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241265983. doi: 10.1177/15330338241265983. Technol Cancer Res Treat. 2024. PMID: 39043046 Free PMC article.
-
Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses.Cochrane Database Syst Rev. 2016 Mar 1;3(3):CD010360. doi: 10.1002/14651858.CD010360.pub2. Cochrane Database Syst Rev. 2016. PMID: 26930463 Free PMC article.
-
Monitoring strategies for clinical intervention studies.Cochrane Database Syst Rev. 2021 Dec 8;12(12):MR000051. doi: 10.1002/14651858.MR000051.pub2. Cochrane Database Syst Rev. 2021. PMID: 34878168 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
