Impact of Adjuvant Chemotherapy Following Neoadjuvant Concurrent Chemoradiotherapy and Total Mesorectal Excision in Rectal Cancer
- PMID: 40579008
- PMCID: PMC12223617
- DOI: 10.21873/invivo.14027
Impact of Adjuvant Chemotherapy Following Neoadjuvant Concurrent Chemoradiotherapy and Total Mesorectal Excision in Rectal Cancer
Abstract
Background/aim: This study investigated the efficacy of adjuvant chemotherapy (ACT) after total mesorectal excision (TME) for rectal cancer in patients who responded well to neoadjuvant concurrent chemoradiotherapy (nCCRT).
Patients and methods: This retrospective study included patients with rectal cancer treated at Taipei Veterans General Hospital (2009-2017). Among 302 patients who underwent nCCRT and TME, 178 good responders [pathologic complete response (pCR), pT1, or pT2] were analyzed. Patients were grouped based on ACT administration. Primary outcomes included disease-free survival (DFS) and recurrence. Kaplan-Meier survival analysis and Cox proportional hazards regression were used to assess ACT efficacy.
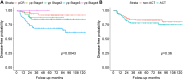
Results: The ACT group (n=96) had poorer baseline disease characteristics, including higher initial clinical T and N stages. However, recurrence rates did not differ significantly between ACT and non-ACT groups (23.2% vs. 17.7%, p=0.271). DFS curves showed no significant difference between ACT and non-ACT groups (p=0.360). Multivariable analysis confirmed that ACT was not significantly associated with DFS [adjusted hazard ratio (aHR)=0.76, 95% confidence interval (CI)=0.37-1.59]. However, the advanced surgical pT stage (pT3-pT4) was an independent predictor of recurrence (aHR=3.24, 95%CI=1.01-10.38, p=0.047).
Conclusion: The role of ACT remains inconclusive after TME for rectal cancer in patients who respond well to nCCRT. Surgical pT stage, particularly pT3 and pT4, remain a significant predictor of recurrence, emphasizing its importance in risk stratification.
Keywords: Rectal cancer; adjuvant chemotherapy (ACT); neoadjuvant concurrent chemoradiotherapy (nCCRT); total mesorectal excision (TME).
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
All the Authors have no conflicts of interest to declare in relation to this manuscript.
Figures
Similar articles
-
Prognostic factors for local recurrence in patients with rectal cancer submitted to neoadjuvant chemoradiotherapy and total mesorectal excision.Clinics (Sao Paulo). 2024 Aug 9;79:100464. doi: 10.1016/j.clinsp.2024.100464. eCollection 2024. Clinics (Sao Paulo). 2024. PMID: 39126876 Free PMC article.
-
Tailored selection of the interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer: analysis based on the pathologic stage or chemoradiation response.J Cancer Res Clin Oncol. 2015 Apr;141(4):719-28. doi: 10.1007/s00432-014-1843-8. Epub 2014 Oct 9. J Cancer Res Clin Oncol. 2015. PMID: 25296560 Free PMC article.
-
Postoperative adjuvant chemotherapy in rectal cancer operated for cure.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004078. doi: 10.1002/14651858.CD004078.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419291 Free PMC article.
-
Neoadjuvant rectal-tumor regression grade combined score as surrogate endpoint for disease-free survival in locally advanced rectal cancer patients after neoadjuvant chemoradiotherapy.Oncologist. 2025 Jun 4;30(6):oyaf124. doi: 10.1093/oncolo/oyaf124. Oncologist. 2025. PMID: 40549045 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264 (11):1444–50. - PubMed
-
- Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA, Twito DI, Morton RF, Veeder MH, Witzig TE, Cha S, Vidyarthi SC. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–715. doi: 10.1056/NEJM199103143241101. - DOI - PubMed
-
- Wee CW, Kang HC, Wu HG, Chie EK, Choi N, Park JM, Kim JI, Huang CM, Wang JY, Ng SY, Goodman KA. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta-analysis and pooled-analysis of acute toxicity. Japanese J Clin Oncol. 2018;48(5):458–466. doi: 10.1093/jjco/hyy029. - DOI - PubMed
-
- Parulekar W, de Marsh RW, Wong R, Mendenhall W, Davey P, Zlotecki R, Berry S, Rout WR, Bjarnason GA. Phase I study of 5-fluorouracil and leucovorin by continuous infusion chronotherapy and pelvic radiotherapy in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58(5):1487–1495. doi: 10.1016/j.ijrobp.2003.09.073. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources