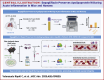
Sodium-Glucose Cotransporter Inhibition Preserves Apolipoprotein M During Acute Inflammation in Mice and Humans
- PMID: 40579057
- PMCID: PMC12277633
- DOI: 10.1016/j.jacadv.2025.101839
Sodium-Glucose Cotransporter Inhibition Preserves Apolipoprotein M During Acute Inflammation in Mice and Humans
Abstract
Background: Sodium-glucose cotransporter inhibitors (SGLT2is) reduce inflammation and maintain vascular integrity. Apolipoprotein M (ApoM) is crucial for vascular integrity via sphingosine-1-phosphate (S1P) signaling and is inversely linked with mortality in sepsis and COVID-19.
Objectives: The authors tested if SGLT2i (dapagliflozin [Dapa]) increases ApoM in mice using lipopolysaccharide (LPS) and in humans with COVID-19.
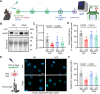
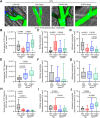
Methods: Diet-induced obese mice (n = 14-15/group), proximal tubule-specific knockout of the multiligand protein receptor Lrp2 (Lrp2KO) mice (n = 5-8/group), Ly6G-Cre LoxP-STOP-TdTomato mice (n = 3-5/group), ApomKO mice (n = 3-5/group), and ApomTG mice (n = 3-5/group) were randomized to receive either vehicle or Dapa (1.25 mg/kg daily) for 4 days before LPS (10 mg/kg IP). Outcomes included ApoM protein levels (Western and enzyme-linked immunosorbent assay) and intravital microscopy to assess endothelial leak and neutrophil behavior. Plasma samples from ACTIV-4a participants (standard of care, n = 37; standard of care + SGLT2i, n = 15) were analyzed for circulating ApoM by enzyme-linked immunosorbent assay. Statistical analyses included two-way analysis of variance for mice and t-test or Mann-Whitney test for humans.
Results: Dapa restored circulating ApoM levels in LPS-treated mice (0.017 vs 0.035 [a.u./μL], P = 0.0489) and increased ApoM levels in patients randomized to SGLT2i (0.5240 vs 0.6537 [μM], P = 0.0101). LRP2 knockout blocked Dapa's effect on ApoM. In vitro, Dapa stimulated Lrp2-dependent uptake of ApoM-GFP. Dapa attenuated vascular leak induced by LPS in an ApoM-dependent manner.
Conclusions: SGLT2i maintains Lrp2 levels, preserving ApoM and promoting endothelial barrier integrity in acute inflammation, indicating a novel protective mechanism of SGLT2i through ApoM preservation.
Keywords: LRP2; SGLT2; apolipoprotein M; endothelial vascular integrity.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures Dr Javaheri was supported by NHLBI (K08HL138262 and 1R01HL155344), the Children's Discovery Institute of Washington University and St. Louis Children's Hospital (MC-FR-2020-919), the Diabetes Research Center at Washington University in St. Louis of the National Institutes of Health (P30DK020579), the Nutrition Obesity Research Center of the National Institutes of Health (P30DK056341), and the Longer Life Foundation. Dr Guo was supported by the American Heart Association Second Century Early Faculty Independence Award (24SCEFIA125647), American Heart Association Postdoctoral Fellowship Award (898679), and Division of Cardiology at Washington University (GF0012995), as well as the Diabetes Research Center at Washington University in St. Louis of the National Institutes of Health (P30DK020579). Dr Lotfinaghsh was supported by the NIH T32 trainee program (HL007081). The research was in part supported by a research grant from AstraZeneca. The research was, in part, funded by the National Institutes of Health (NIH) Agreement 1OT2HL156812 through the National Heart, Lung, and Blood Institute (NHLBI) CONNECTS program. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH. Dr Javaheri has a pending patent for fusion protein nanodiscs for the treatment of heart failure and eye disease, is a member of the scientific advisory board of Mobius Scientific, and receives research funding from Bitterroot Bio, unrelated to the studies in this manuscript. The present study was in part supported by a research grant from AstraZeneca. Dr Kosiborod receives consulting fees/honoraria from Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck, Novo Nordisk, Pharmacosmos, Sanofi-Aventis, and Vifor Pharma and research grants or contracts from AstraZeneca and Boehringer Ingelheim. Drs Esterline and Oscarsson are employees and stockholders of AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. - PubMed
-
- Zelniker T.A., Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:435–447. - PubMed
Grants and funding
- R01 HL146559/HL/NHLBI NIH HHS/United States
- R01 HL155344/HL/NHLBI NIH HHS/United States
- R01 AI181792/AI/NIAID NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 HL153047/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- K08 HL138262/HL/NHLBI NIH HHS/United States
- R01 HL177904/HL/NHLBI NIH HHS/United States
- R01 HL130028/HL/NHLBI NIH HHS/United States
- T32 HL007081/HL/NHLBI NIH HHS/United States
- R01 HL148280/HL/NHLBI NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- S10 OD028597/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

