[ATF3 regulates inflammatory response in atherosclerotic plaques in mice through the NF-κB signaling pathway]
- PMID: 40579127
- PMCID: PMC12204839
- DOI: 10.12122/j.issn.1673-4254.2025.06.03
[ATF3 regulates inflammatory response in atherosclerotic plaques in mice through the NF-κB signaling pathway]
Abstract
Objectives: To investigate the role of activating transcription factor 3 (ATF3) in atherosclerotic plaques for regulating inflammatory responses during atherosclerosis (AS) progression.
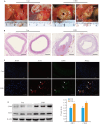
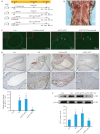
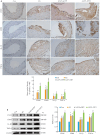
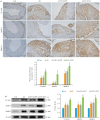
Methods: Human coronary artery specimens from autopsy cases were examined for ATF3 protein expression and localization using immunofluorescence staining and Western blotting. Apolipoprotein E-deficient (ApoE-/-) mouse models of AS induced by high-fat diet (HFD) feeding for 12 weeks were subjected to tail vein injection of adeno-associated virus serotype 9 (AAV9) to knock down ATF3 expression. After an additional 5 weeks of HFD feeding, the mice were euthanized for analyzing structural changes of the aortic plaques, and the expression levels of ATF3, inflammatory factors (CD45, CD68, IL-1β, and TNF-α), and NF-κB pathway proteins (P-IKKα/β and P-NF-κB p65) were detected. In the cell experiment, THP-1-derived foam cells were transfected with an ATF3-overexpressing plasmid or an ATF3-specific siRNA to validate the relationship between ATF3 and NF‑κB signaling.
Results: In human atherosclerotic plaques, ATF3 expression was significantly elevated and partially co-localized with CD68. ATF3 knockout in ApoE-/- mice significantly increased aortic plaque volume, upregulated the inflammatory factors, enhanced phosphorylation of the NF‑κB pathway proteins, and increased the expressions of VCAM1, MMP9, and MMP2 in the plaques. In THP-1-derived foam cells, ATF3 silencing caused activation of the NF‑κB pathway, while ATF3 overexpression suppressed the activity of the NF-κB pathway.
Conclusions: AS promotes ATF3 expression, and ATF3 deficiency exacerbates AS progression by enhancing plaque inflammation via activating the NF-κB pathway, suggesting the potential of ATF3 as a therapeutic target for AS.
目的: 探讨转录激活因子3(ATF3)在动脉粥样硬化斑块内的表达及其调控炎症反应参与动脉粥样硬化(AS)进程的机制。方法: 收集尸检案例中的人冠状动脉标本,免疫荧光和Western blotting检测ATF3蛋白表达及分布;高脂饮食12周后的载脂蛋白E基因敲除(ApoE-/-)小鼠尾静脉注射9型腺相关病毒(AAV9)敲低ATF3的表达,继续高脂喂养5周构建ATF3基因敲除的动脉粥样硬化ApoE-/-小鼠模型。麻醉处死后观察主动脉斑块结构改变,检测斑块内ATF3、炎症相关因子及NF-κB信号通路蛋白表达改变,并在体外构建ATF3的过表达质粒及siRNA干预THP-1诱导的泡沫细胞模型验证ATF3与NF-κB信号通路间关系。结果: 在人冠状动脉粥样硬化斑块内,ATF3的表达增高(P<0.05),且与CD68呈部分共表达;在ATF3基因敲除后,小鼠的主动脉斑块体积增大(P<0.05),斑块内炎症相关因子(CD45、CD68、IL-1β、TNF-α)表达增强(P<0.05),NF-κB信号通路相关蛋白(P-IKKα/β、P-NF-κB p65)表达增高(P<0.05),VCAM1、MMP9及MMP2表达增强(P<0.05);在离体THP-1细胞实验中验证了沉默ATF3后NF-κB信号通路进一步激活,而过表达ATF3后NF-κB信号受到抑制。结论: 动脉粥样硬化诱导ATF3的表达,ATF3的缺乏会促进AS的发生,增强斑块内炎症反应,其机制可能是通过进一步激活NF-κB信号通路导致,ATF3可能是AS潜在的治疗靶点。.
Keywords: activating transcription factor 3; atherosclerosis; inflammatory response; nuclear factor-κB; sudden cardiac death.
Figures



References
-
- Wang XW, Yan KY, Wen CF, et al. Simvastatin combined with resistance training improves outcomes in patients with chronic heart failure by modulating mitochondrial membrane potential and the Janus kinase/signal transducer and activator of transcription 3 signaling pathways[J]. Cardiovasc Ther, 2022, 2022: 8430733. doi: 10.1155/2022/8430733 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
