Immunotherapy for rapid bone marrow conditioning and leukemia depletion that allows efficient hematopoietic stem cell transplantation
- PMID: 40579234
- PMCID: PMC12207149
- DOI: 10.1136/jitc-2025-011888
Immunotherapy for rapid bone marrow conditioning and leukemia depletion that allows efficient hematopoietic stem cell transplantation
Abstract
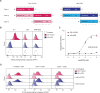
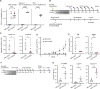
Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure to treat hematopoietic disorders. Current bone marrow conditioning protocols create space for healthy donor stem cells by employing irradiation and/or chemotherapy, but carry severe toxicities, resulting in significant morbidity, mortality and substantial long-term complications. To develop a low-toxicity solution, we generated a bi-specific T-cell engager (BTCE) that targets CD117, an abundantly expressed receptor on hematopoietic stem and progenitor cells (HSPC) and leukemia-initiating cells (LICs). We show that the CD117×CD3 BTCE efficiently depletes in vitro and in vivo HSPCs and LICs. The CD117×CD3 BTCE was not toxic and facilitates highly efficient engraftment of human allogenic donor CD34+cells in humanized mice, thereby restoring hematopoiesis in vivo in both normal and leukemia-bearing humanized mice. We demonstrate here that a potent CD117×CD3 BTCE enables rapid HSCT in both benign and malignant conditions.
Keywords: Bispecific T cell engager - BiTE; Immunotherapy; Leukemia; Pharmacokinetics - PK; Stem cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: SE, PDK, YW and YH are inventors of CD117xCD3 reagent, which is patented (PCT/CN2022/140683, international publication number: WO 2024/131861 A1). YW, YH and JZ were employees of Harbour Biomed at the time this work was performed. Health Holland grant (EMCLSH20003) includes in-kind contribution of Harbour Biomed and Erasmus MC.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
