Effectiveness of Bimekizumab in Psoriatic Arthritis: A Multicenter Real-Life Study With Clinical and Ultrasound Evaluation in the Lazio Region, Italy
- PMID: 40579388
- PMCID: PMC12508706
- DOI: 10.1111/ijd.17927
Effectiveness of Bimekizumab in Psoriatic Arthritis: A Multicenter Real-Life Study With Clinical and Ultrasound Evaluation in the Lazio Region, Italy
Abstract
Background: Bimekizumab, a monoclonal antibody targeting interleukin (IL)-17A and IL-17F, has shown efficacy in psoriatic arthritis (PsA) clinical trials, but real-world data on its effectiveness are limited. This study aimed to evaluate its real-world effectiveness in PsA patients treated in dermatologic settings.
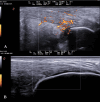
Methods: We conducted a multicenter, prospective, observational study in dermatology units across Lazio, Italy, including 20 patients with psoriasis and PsA treated with bimekizumab. Rheumatologic assessments were performed at baseline and after 16 weeks, evaluating tender and swollen joint counts, the Visual Analog Scale (VAS) pain score, the Disease Activity in Psoriatic Arthritis (DAPSA) score, and the Leeds Enthesitis Index (LEI). Ultrasound evaluations (US) of joints and entheses were conducted at baseline and Week 16.
Results: Twenty patients were enrolled, including 10 bio-experienced patients. At 16 weeks, significant improvements were observed in tender joint count (3.65 to 2.15, p = 0.004), swollen joint count (1.35 to 0.55, p = 0.028), VAS pain score (6.35 to 3.35, p < 0.001), DAPSA (22.71 to 8.67, p < 0.001), and LEI (0.85 to 0.25, p = 0.004). US revealed a decrease in the number of patients with power Doppler (PD) positive joints (45% to 25%) and a reduction in synovial PD score (2.26 to 0.81, p = 0.002). The number of patients with US-positive enthesitis decreased (50% to 30%), with a significant reduction in the enthesis PD score (1.00 to 0.25, p = 0.047). Four patients (20%) developed mild candidiasis, with no treatment discontinuation.
Conclusions: Bimekizumab demonstrated significant, rapid improvements in real-world PsA patients, including those with prior biologic failures. Its favorable safety profile and effectiveness highlight its potential as a valuable treatment option for PsA.
Keywords: Interleukin‐17; bimekizumab; biologic therapies; psoriasis; psoriatic arthritis; real‐world effectiveness; ultrasound.
© 2025 The Author(s). International Journal of Dermatology published by Wiley Periodicals LLC on behalf of the International Society of Dermatology.
Conflict of interest statement
Giacomo Caldarola has received consulting fees, honoraria, and support for attending meetings from AbbVie, Lilly, Janssen, UCB, Novartis, and Leopharma; Diego Orsini has been a speaker and/or consultant for AbbVie, Almirall, LeoPharma, UCB, Bristol‐MyersSquibb, and Boehringer Ingelheim. All other authors declare that they have no conflicts of interest relevant to this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

