Reshaping the tumor microenvironment of cold soft-tissue sarcomas with anti-angiogenics: a phase 2 trial of regorafenib combined with avelumab
- PMID: 40579407
- PMCID: PMC12205094
- DOI: 10.1038/s41392-025-02278-9
Reshaping the tumor microenvironment of cold soft-tissue sarcomas with anti-angiogenics: a phase 2 trial of regorafenib combined with avelumab
Abstract
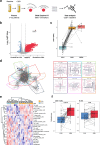
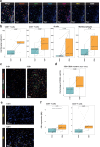
The majority of sarcomas are under the influence of a tumor microenvironment that dampens immune activity, resulting in resistance to monoclonal antibodies targeting immune checkpoints and reduced clinical effectiveness. Preclinical studies indicate that targeting abnormal neoangiogenesis by inhibiting vascular endothelial growth factor receptor (VEGFR) can alter the TME, thereby promoting T cell infiltration and increasing tumor immunogenicity. The REGOMUNE study, a phase II clinical trial, assessed the therapeutic combination of regorafenib, a multityrosine kinase inhibitor that targets VEGFR2 and the PD-L1 blocker avelumab, in individuals with advanced "cold" STS characterized by a lack of mature tertiary lymphoid structures (mTLS). Forty-nine mTLS-negative STS patients were enrolled, including leiomyosarcoma (45%), synovial sarcoma (18%), and other subtypes. The objective response rate was 11.0% (95% CI: 4.0% - 22.0%), with median progression-free survival and overall survival of 1.8 months (95% CI, 1.7-3.5 months) and 15.1 months, respectively. Frequent adverse events included grade 1 or 2 palmar-plantar erythrodysesthesia, fatigue, and diarrhea. On-treatment multiplex immunofluorescence analysis revealed significant increases in CD8 + T cell and B cell infiltration and PD1 expression on immune cells. Plasma analysis indicated significant upregulation of soluble PD-L1 (sPD-L1) levels and tryptophan consumption. Overall, these results indicate that anti-angiogenic therapy modulates the tumor microenvironment in patients with cold STS and highlight the need for complementary strategies to enhance the functional activity of immune cells in this particular setting. Clinical trial registration number: NCT03475953.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.I.: research grant (AstraZeneca, Bayer, BMS, Merck, MSD, Pharmamar). J.P.G., C.R., and A.B.: employees of Explicyte. The other co-authors have nothing to disclose. The other authors declare that they have no competing interests. Ethics approval: This study was approved by the Comité de Protection des Personnes (CPP) Sud-Ouest et Outre Mer III (Bordeaux, France) according to good clinical practices and applicable laws and regulations. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
Figures


References
-
- Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature577, 556–560 (2020). - PubMed
-
- Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol.19, 441–457 (2022). - PubMed
-
- Italiano, A. et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat. Med.28, 1199–1206 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

