The synaptonemal complex central element SCEP3 interlinks synapsis initiation and crossover formation in Arabidopsis thaliana
- PMID: 40579437
- PMCID: PMC12283363
- DOI: 10.1038/s41477-025-02030-9
The synaptonemal complex central element SCEP3 interlinks synapsis initiation and crossover formation in Arabidopsis thaliana
Abstract
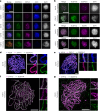
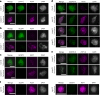
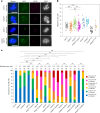
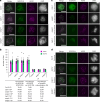
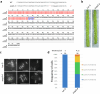
The synaptonemal complex (SC) forms between homologous chromosomes during meiosis. In Arabidopsis thaliana, its central region (CR) is composed of the transverse filament protein ZYP1 and the central element proteins SCEP1 and SCEP2. Here we identify SCEP3 as a CR protein that is evolutionarily conserved across plant species. SCEP3 spatiotemporally overlaps with other CR proteins and localizes to the SC CR. The loss of SCEP3 prevents SC assembly, abolishes crossover (CO) assurance and interference, and eliminates sex-specific differences in CO rates (heterochiasmy) through increased CO in females. SCEP3 is required for a subset of COs in SC-deficient mutants, such as zyp1. Although SCEP3 physically interacts with ZYP1, it loads independently of other CR proteins. We propose that SCEP3 may associate with certain recombination intermediates, stabilizing them and/or recruiting additional factors, such as ZYP1, to a subset of these intermediates, thereby promoting and interlinking SC assembly and CO formation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Zickler, D. & Kleckner, N. Meiosis: dances between homologs. Annu. Rev. Genet.57, 1–63 (2023). - PubMed
-
- de Massy, B. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet.47, 563–599 (2013). - PubMed
-
- Mercier, R., Mezard, C., Jenczewski, E., Macaisne, N. & Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol.66, 297–327 (2015). - PubMed
-
- Börner, G. V., Kleckner, N. & Hunter, N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell117, 29–45 (2004). - PubMed
-
- Snowden, T., Acharya, S., Butz, C., Berardini, M. & Fishel, R. hMSH4–hMSH5 recognizes Holliday junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol. Cell15, 437–451 (2004). - PubMed
MeSH terms
Substances
Grants and funding
- 354617974/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 543670370/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 949618/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- CSC202103250012/CSC | Chinese Government Scholarship
LinkOut - more resources
Full Text Sources

