AAV mini-dystrophin gene therapy for Duchenne muscular dystrophy: a phase 1b trial
- PMID: 40579547
- PMCID: PMC12353823
- DOI: 10.1038/s41591-025-03750-3
AAV mini-dystrophin gene therapy for Duchenne muscular dystrophy: a phase 1b trial
Abstract
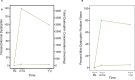
Gene therapy represents a promising approach for Duchenne muscular dystrophy (DMD), a rare X-linked genetic muscle disease. Fordadistrogene movaparvovec (PF-06939926) is an adeno-associated virus serotype 9 gene therapy containing a miniaturized dystrophin being developed for DMD, which aims to restore functional protein to muscle. We present 1-year data from ambulatory and nonambulatory participants in a phase 1b, multicenter, single-arm, open-label trial. Pediatric ambulatory male participants with a genetic DMD diagnosis and receiving stable glucocorticoids received a single intravenous low-dose (n = 3) or high-dose (n = 16) fordadistrogene movaparvovec. The primary endpoint was safety and tolerability at 1 year after dosing. In the ambulatory group, mean ± s.d. age at dosing was 8.6 ± 1.6 years. The most common treatment-emergent adverse events in the ambulatory group were vomiting (n = 15), nausea (n = 10), thrombocytopenia (n = 9), pyrexia (n = 9), decreased appetite (n = 8), fatigue (n = 7) and headache (n = 7). Three treatment-related serious adverse events occurred after dosing (dehydration, acute kidney injury, thrombocytopenia; all resolved within 15 days). In a small nonambulatory group (n = 3), mean ± s.d. age at dosing was 15.1 ± 1.0 years. The most common treatment-emergent adverse events were nausea (n = 3), vomiting (n = 3) and headache (n = 3); two severe treatment-related adverse events (hemolytic uremic syndrome and fatal cardiogenic shock) were observed. In the high-dose ambulatory group, the secondary endpoint of mini-dystrophin quantification showed robust expression. Mean (95% confidence interval) percent of mini-dystrophin-positive fibers for baseline, 2 months and 1 year were 0.1% (0.1-0.2), 20.3% (12.2-29.3) and 34.8% (21.1-49.8), respectively. At the 1-year time point of primary completion, fordadistrogene movaparvovec demonstrated an acceptable safety profile in the ambulatory population. Larger trials are needed to assess the efficacy of the gene therapy in DMD. ClinicalTrials.gov registration no. NCT03362502 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.J.B. has received support from or has served as a consultant for Sarepta Pharmaceuticals, Scholar Rock, AveXis, Pfizer, Biogen, Reata and Aavanti. P.B.S. has received contracted research support from Pfizer; his institution has received contracted research funding from Sarepta, Solid Biosciences, ReveraGen, PTC Therapeutics, Astellas Gene Therapies, Novartis Gene Therapies, Santhera, Biogen, AMO Pharma and Sanofi. He has served on advisory boards for Sarepta, Alexion, Argenx, Biogen and Genentech, and has received speaker fees from Alexion, Catalyst, Grifols, Genentech, CSL Behring, Biogen and Argenx. H.L., K.A.R., M.D., S.N., D.I.L. and P.F.S. are employees of Pfizer and hold stock or stock options. M.B. and T.G.M. were employees of Pfizer and held stock or stock options at the time of the study. M.B. and T.G.M. are also named on a patent (no. WO2020261178A1) applied for ‘Methods of treating Duchenne muscular dystrophy using AAV mini-dystrophin gene therapy’ but receive no direct financial benefit. B.A.B. (currently retired) was an employee of Pfizer at the time of the study. E.C.S. has received partial salary support from Pfizer for his role as the principal investigator of the study and has served as a paid consultant for Pfizer, Sarepta, NSPharma, Lilly, REGENXBIO, Entrada, Solid Biosciences and Edgewise Therapeutics.
Figures




References
-
- Hoffman, E. P., Brown, R. H. Jr. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell51, 919–928 (1987). - PubMed
-
- Beekman, C., Janson, A. A., Baghat, A., van Deutekom, J. C. & Datson, N. A. Use of capillary Western immunoassay (Wes) for quantification of dystrophin levels in skeletal muscle of healthy controls and individuals with Becker and Duchenne muscular dystrophy. PLoS ONE13, e0195850 (2018). - PMC - PubMed
-
- Mercuri, E., Bönnemann, C. G. & Muntoni, F. Muscular dystrophies. Lancet394, 2025–2038 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

