Macluraparishin C Enhances Neuroprotection Against Oxidative Stress-Induced Neurodegeneration by Activating the Antioxidant/MAPK Signaling Pathway
- PMID: 40579647
- PMCID: PMC12433353
- DOI: 10.1007/s12035-025-05139-2
Macluraparishin C Enhances Neuroprotection Against Oxidative Stress-Induced Neurodegeneration by Activating the Antioxidant/MAPK Signaling Pathway
Abstract
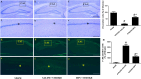
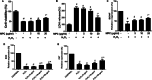
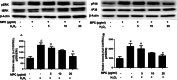
Oxidative stress plays a critical role in the development and progression of neurodegenerative diseases through the induction of neuronal injury and apoptosis. Macluraparishin C (MPC) is a novel parishin compound with identified potent neuroprotection, whose molecular mechanisms have not been clarified yet. The present research aims to explore the neuroprotective function of MPC in the context of the bilateral common carotid artery (BCCA) occlusion-induced transient global cerebral ischemia (tGCI) in gerbils, along with hydrogen peroxide (H2O2)-induced oxidative stress in SH-SY5Y cells. The neuroprotective capability of MPC was appraised by conducting several assays like cell viability, lactase dehydrogenase (LDH) assay, quantitative real-time PCR, Western blot, immunohistochemistry (IHC), cresyl violet (CV), and fluoro-jade B (FJB) staining. The pretreatment with MPC has significantly reduced the number of microglia and astrocytes present in the hippocampal CA1 area, as well as the cell death of neurons in gerbils after tGCI. Moreover, MPC pretreatment downregulates the protein expressions of the MAPK cascade, including ERK, JNK, and p38, and regulates antioxidant enzymes, such as SOD2, GPX1, GPX4, and CAT, in both hippocampi and SH-SY5Y cells. In the SH-SY5Y cell line, MPC decreased neurotoxicity induced by H2O2 by lessening LDH release and enhancing neuronal stress marker genes like brain-derived neurotrophic factor (BDNF). These findings suggest that MPC may protect neurons by boosting antioxidant/MAPK pathways, showing potential for treating oxidative stress-related neurodegenerative diseases.
Keywords: Antioxidant; MAPK; Macluraparishin C; Neurodegeneration; Oxidative stress; Transient global cerebral ischemia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: The study protocols were carried out following relevant guidelines and regulations approved by the animal welfare regulations of the Institutional Animal Care and Use Committee (approval no. CBNU-2020–003) of the Jeonbuk National University Laboratory Animal Center in South Korea and in compliance with the ARRIVE guidelines. Consent for Publication: Not applicable. Conflict of Interest: The authors declare no competing interests.
Figures




References
-
- Harukuni I, Bhardwaj A (2006) Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 24(1):1–21 - PubMed
-
- Yoshikawa T, Naito Y (2002) What is oxidative stress? Japan medical association journal 45(7):271–276
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

