Tracing Early Migratory Neurons in the Developing Nose Using Contactin-2 (Cntn2) CreERT2
- PMID: 40580015
- PMCID: PMC12205475
- DOI: 10.1002/dvg.70021
Tracing Early Migratory Neurons in the Developing Nose Using Contactin-2 (Cntn2) CreERT2
Abstract
Neuronal migration during embryonic development is a fundamental process. In the developing nose of rodents, neurons that form during early neurogenic waves in the olfactory placode leave this structure to migrate toward or into the developing brain as part of the migratory mass. This mass includes gonadotropin-releasing hormone-1 (GnRH-1) neurons, pioneer/terminal nerve (TN) neurons, as well as neural crest-derived olfactory glial cells called olfactory ensheathing cells. There have been a limited number of molecular markers available to effectively trace and functionally manipulate the early migratory neurons that originate in the olfactory region. Contactin-2 (Cntn2), also known as transiently expressed axonal surface glycoprotein-1 (TAG-1), has been used to label various developing neuronal populations, including the commissural neurons of the spinal cord, motor neurons, and TN neurons. Previous single-cell RNA sequencing analyses of the developing olfactory system have identified Cntn2 expression in the TN, suggesting that Cntn2 is a suitable molecular marker for studying nasal migratory neurons. To trace Cntn2 expression in the developing olfactory system, we generated an inducible Cntn2CreERT2 mouse line. In this study, we outline how this mouse line can serve as an effective tool for time-controlled chimeric manipulation of specific neuronal populations of interest.
Keywords: Contactin‐2 (Cntn2); GnRH neurons; migratory mass; olfactory development; spinal cord; terminal nerve.
© 2025 The Author(s). genesis published by Wiley Periodicals LLC.
Figures
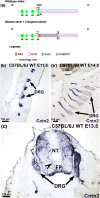


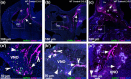
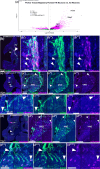


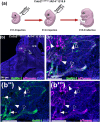

Update of
-
Tracing Early Migratory Neurons in the Developing Nose Using Contactin-2 (Cntn2)CreERT2.bioRxiv [Preprint]. 2025 Apr 1:2025.03.27.645843. doi: 10.1101/2025.03.27.645843. bioRxiv. 2025. Update in: Genesis. 2025 Aug;63(4):e70021. doi: 10.1002/dvg.70021. PMID: 40236046 Free PMC article. Updated. Preprint.
References
-
- Casoni, F. , Malone S. A., Belle M., et al. 2016. “Development of the Neurons Controlling Fertility in Humans: New Insights From 3D Imaging and Transparent Fetal Brains.” Development 143: 3969–3981. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

