Observational Study of the Impact of Infusion Set Replacement in Insulin Pump Users on Glycemic Management
- PMID: 40580090
- PMCID: PMC12206229
- DOI: 10.1177/19322968251345837
Observational Study of the Impact of Infusion Set Replacement in Insulin Pump Users on Glycemic Management
Abstract
Background: Although several studies have evaluated the impact of prolonged infusion set use in insulin pump users on glycemic management with the use of continuous glucose monitoring (CGM), real-world assessments without intervention have been unavailable.
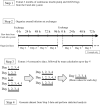
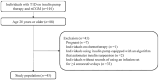
Methods: This retrospective observational study recruited individuals with type 1 diabetes who received insulin pump therapy with real-time CGM. Insulin pump and CGM logs were extracted from the Medtronic CareLink system, and a dataset was constructed programmatically, counting tracking days from infusion set replacement every 24 hours up to day 4. The primary outcome was mean sensor glucose (SG) level, and the impact of infusion set usage duration on glycemic management was assessed.
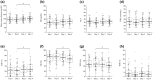
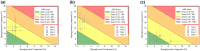
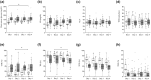
Results: The study enrolled 45 individuals with a median age of 40 (interquartile range = 32-51) years and median body mass index of 22.5 (21.2-23.8) kg/m². Mean SG was significantly higher on day 4 (median of 151.9 [136.5-173.7] mg/dL) than on day 2 (144.4 [124.0-162.9] mg/dL, P = .024). Similarly, time above range (TAR), time in range (TIR), and time in tight range (TITR) had worsened on day 4 compared with day 2. The TAR increased from a median of 22.0% (7.3%-35.8%) on day 2 to 27.6% (17.7%-44.9%) on day 4, whereas TIR decreased from 74.2% (59.9%-87.1%) to 66.5% (52.0%-79.8%) and TITR decreased from 47.6% (37.1%-67.5%) to 42.2% (34.6%-54.1%).
Conclusions: Our evaluation of the real-world impact of prolonged infusion set use revealed an association between longer use and worsening of glycemic management.
Keywords: continuous glucose monitoring; infusion set; insulin pump therapy; observational study; type 1 diabetes.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: W.O. has received lecture fees from Nippon Boehringer Ingelheim Co Ltd, Sumitomo Pharma Co Ltd, and Novo Nordisk Pharma Ltd; research expenses (including for contracted research, joint research, and clinical trials) and grants from Abbott Diabetes Care UK Ltd, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co Ltd, Novo Nordisk Pharma Ltd, Noster Inc, Teijin Pharma Ltd, and Sumitomo Pharma Co Ltd; and scholarship donations from Sumitomo Pharma Co Ltd, Teijin Pharma Ltd, and Takeda Pharmaceutical Co Ltd. Y.H. has received lecture fees from Eli Lilly Japan K.K., Sanofi, Terumo Corp, Sumitomo Pharma Co Ltd, Novo Nordisk Pharma Ltd, and Abbott Japan LLC; research expenses (including for contracted research, joint research, and clinical trials) and grant from Sumitomo Pharma Co Ltd, Kyowa Kirin Co Ltd, Medtronic Japan Co Ltd, and Nippon Boehringer Ingelheim Co Ltd; and scholarship donations from Abbott Japan LLC. All remaining authors declare that there is no conflict of interest.
Figures
Similar articles
-
Continuous glucose monitoring systems for type 1 diabetes mellitus.Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD008101. doi: 10.1002/14651858.CD008101.pub2. Cochrane Database Syst Rev. 2012. PMID: 22258980 Free PMC article.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.Health Technol Assess. 2004 Oct;8(43):iii, 1-171. doi: 10.3310/hta8430. Health Technol Assess. 2004. PMID: 15488165
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Impact of the initiation of isCGM soon after type 1 diabetes mellitus diagnosis in adults on glycemic indices and fear of hypoglycemia: a randomized controlled trial.Front Endocrinol (Lausanne). 2025 Jan 9;15:1503891. doi: 10.3389/fendo.2024.1503891. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39850477 Free PMC article. Clinical Trial.
-
Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review.J Manag Care Pharm. 2012 Aug;18(6 Suppl):S1-17. doi: 10.18553/jmcp.2012.18.s6-a.1. J Manag Care Pharm. 2012. PMID: 22984955 Free PMC article.
References
-
- Wheeler BJ, Heels K, Donaghue KC, Reith DM, Ambler GR. Insulin pump-associated adverse events in children and adolescents–a prospective study. Diabetes Technol Ther. 2014;16(9):558-562. - PubMed
LinkOut - more resources
Full Text Sources

