Functionalization of Electron-Rich Secondary Benzyl Alcohols in HFIP: From Thioethers to Trisubstituted Methanes
- PMID: 40580135
- PMCID: PMC12261332
- DOI: 10.1021/acs.joc.5c00900
Functionalization of Electron-Rich Secondary Benzyl Alcohols in HFIP: From Thioethers to Trisubstituted Methanes
Abstract
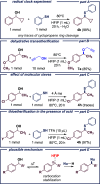
To date, C-S bond formation via dehydration of electron-rich secondary benzylic alcohols has been limited to catalytic approaches. In this work, we developed an efficient method for synthesizing various thioethers by employing hexafluoroisopropanol (HFIP) as a solvent. This approach also enables the functionalization of secondary benzyl alcohols with other important organic molecules, such as allylsilanes, activated arenes, and indoles, leading to various trisubstituted methanes, including 14 novel products in total.
Figures
Similar articles
-
Light-Driven C(sp3)-C(sp3) Bond Functionalizations Enabled by the PCET Activation of Alcohol O-H Bonds.Acc Chem Res. 2025 Jul 1;58(13):2061-2071. doi: 10.1021/acs.accounts.5c00246. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40511721
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Ghosh S., Patra K., Baidya M.. Allure of HFIP in Unsaturated Carbon–Carbon Bond Functionalization. Eur. J. Org. Chem. 2024;27(12):e202301321. doi: 10.1002/ejoc.202301321. - DOI
LinkOut - more resources
Full Text Sources