NR4A2 attenuates early brain injury after intracerebral hemorrhage by promoting M2 microglial polarization via TLR4/TRAF6/NF-κB pathway inhibition
- PMID: 40580316
- PMCID: PMC12206217
- DOI: 10.1007/s00018-025-05755-0
NR4A2 attenuates early brain injury after intracerebral hemorrhage by promoting M2 microglial polarization via TLR4/TRAF6/NF-κB pathway inhibition
Abstract
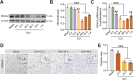
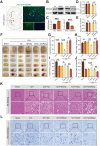
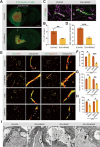
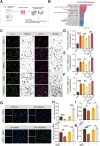
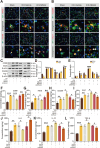
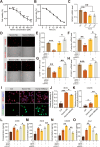
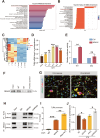
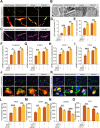
In the early stage of intracerebral hemorrhage (ICH), rebleeding occurs when blood from the initial hematoma permeates the surrounding brain parenchyma through the disrupted blood-brain barrier (BBB), exacerbating brain injury. Neuroinflammation is a critical driver of the pathological processes underlying this phenomenon. Research on microglia near early hematomas revealed that promoting the transition of microglia to the M2 phenotype could mitigate perihematomal inflammatory damage. Recent studies have shown that the nuclear receptor-related 1 protein (NR4A2) can regulate microglial function and inhibit inflammation. However, the functions of NR4A2 in the development of ICH are still unclear. In this study, we explored the potential protective effect and mechanism of NR4A2 in ICH models. Our results demonstrated that the expression of NR4A2 was significantly decreased in both ICH rats and cell models. Increasing NR4A2 activity could effectively decrease the hematoma volume, improve the neurological prognosis and alleviate perihematomal BBB damage. In vivo and in vitro experiments revealed that NR4A2 inhibited perihematomal inflammatory damage by driving microglial polarization toward the anti-inflammatory M2 phenotype. Mechanistically, NR4A2 targeted TLR4 and inhibited the TRAF6/NF-κB pathway, thereby promoting M2 microglial polarization, reducing inflammatory cell extravasation and maintaining the integrity of the BBB. Conversely, the protective effects of NR4A2 were negated when CRX-527 (a TLR4 agonist) was introduced. These findings suggest that NR4A2 represents a promising therapeutic target for ICH.
Keywords: Blood–brain barrier; Microglial polarization; NR4A2; Neuroinflammation; TLR4; TRAF6/NF-κB.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Human umbilical cord mesenchymal stem cell-derived exosomes inhibit inflammation and fibrotic scar formation after intracerebral hemorrhage.Mol Cell Biochem. 2025 Aug;480(8):4829-4847. doi: 10.1007/s11010-025-05259-2. Epub 2025 Apr 25. Mol Cell Biochem. 2025. PMID: 40279088 Free PMC article.
-
Inhibition of P2Y6 Receptor-Mediated Microglia Phagocytosis Aggravates Brain Injury in Mice of Intracerebral Hemorrhage.Cell Mol Neurobiol. 2025 Jul 8;45(1):67. doi: 10.1007/s10571-025-01573-x. Cell Mol Neurobiol. 2025. PMID: 40627214 Free PMC article.
-
Deacetylase SIRT2 Inhibition Promotes Microglial M2 Polarization Through Axl/PI3K/AKT to Alleviate White Matter Injury After Subarachnoid Hemorrhage.Transl Stroke Res. 2025 Aug;16(4):1075-1093. doi: 10.1007/s12975-024-01282-5. Epub 2024 Aug 5. Transl Stroke Res. 2025. PMID: 39103659
-
Immune-mediated disruption of the blood-brain barrier after intracerebral hemorrhage: Insights and potential therapeutic targets.CNS Neurosci Ther. 2024 Jul;30(7):e14853. doi: 10.1111/cns.14853. CNS Neurosci Ther. 2024. PMID: 39034473 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M et al (2022) Post-stroke cognitive impairment and dementia [J]. Circ Res 130(8):1252–1271 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

