Protocol to study DNA strand breaks during development and apoptosis using in situ nick translation in Drosophila
- PMID: 40580472
- PMCID: PMC7617862
- DOI: 10.1016/j.xpro.2025.103921
Protocol to study DNA strand breaks during development and apoptosis using in situ nick translation in Drosophila
Abstract
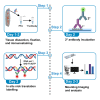
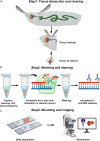
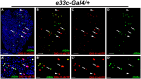
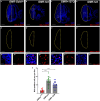
Cellular stress causes DNA strand breaks that are typically repaired to maintain homeostasis and regulate cell fate. However, unrepaired DNA breaks can be lethal, leading to cell death. Here, we present a protocol to study DNA strand breaks in Drosophila during development and apoptosis using in situ nick translation. We describe the steps for labeling DNA strand breaks using digoxigenin (DIG)-labeled nucleotide (DIG-11-dUTP) and visualizing them with anti-DIG immunostaining. We then detail procedures for mounting, imaging, and analysis. For complete details on the use and execution of this protocol, please refer to Maurya et al.1 and Rigby et al.2.
Keywords: Cell Biology; Developmental biology; Microscopy; Model Organisms.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Kelly R.B., Cozzarelli N.R., Deutscher M.P., Lehman I.R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J. Biol. Chem. 1970;245:39–45. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

