Cord blood levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 correlate with perinatal brain development in fetal congenital heart disease
- PMID: 40580544
- PMCID: PMC12317303
- DOI: 10.1002/uog.29271
Cord blood levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 correlate with perinatal brain development in fetal congenital heart disease
Abstract
Objectives: Neonates with critical congenital heart disease (CCHD) are at risk for adverse early brain development and long-term neurodevelopmental sequelae. Insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) are essential contributors to brain growth and maturation. The present study aimed to compare cord blood levels of IGF-1 and IGFBP-3 between neonates with CCHD and healthy controls, and to explore their association with perinatal brain development.
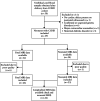
Methods: This was a prospective, observational study conducted at Wilhelmina Children's Hospital, Utrecht, The Netherlands, between June 2019 and March 2022. The study cohort comprised term neonates diagnosed prenatally with a severe cardiac defect on ultrasound, for which surgical repair within the first month after birth was anticipated. IGF-1 and IGFBP-3 levels were measured from cord blood samples obtained directly after birth in term neonates with CCHD and healthy control neonates. All cases were stratified into two subgroups according to the predicted cerebral oxygen delivery (COD) in utero (i.e. normal or lower), based on the expected impact of the cardiac diagnosis on COD. In cases with CCHD, fetal and preoperative neonatal brain magnetic resonance imaging (MRI) scans were performed. Regional and total brain volumes were assessed, after adjustment for sex and postmenstrual age at MRI, using linear regression equations.
Results: Cord blood samples were collected from 39 neonates with CCHD and 20 healthy controls. IGFBP-3 levels were significantly lower in cases of CCHD vs healthy controls (median, 0.89 mg/L vs 1.09 mg/L; P = 0.027). When comparing the two subgroups of predicted COD, cases with lower COD (n = 30) displayed reduced IGF-1 (median, 9.7 nmol/L vs 11.7 nmol/L; P = 0.003) and IGFBP-3 (median, 0.88 mg/L vs 1.07 mg/L; P = 0.005) levels compared to those with normal COD (n = 29). IGF-1 levels were associated positively with neonatal cortical gray matter (R2, 0.13), deep gray matter (R2, 0.15) and total brain (R2, 0.10) volumes (all P < 0.05). Positive associations were identified between IGFBP-3 and neonatal cortical gray matter (R2, 0.07) as well as cerebellar (R2, 0.11) volumes (both P < 0.05). Furthermore, IGF-1 was correlated positively (r = 0.47; P = 0.039) with fetal-to-neonatal cortical gray matter growth per week.
Conclusions: IGFBP-3 levels were demonstrated to be reduced in neonates with CCHD, and cases with lower predicted COD demonstrated decreased levels of IGF-1 and IGFBP-3. In addition, lower IGF-1 and IGFBP-3 levels at birth were associated with reduced early volumetric brain development. These results indicate that a compromised oxygenation profile in utero in fetuses with CCHD may influence the IGF axis, possibly contributing to impaired brain growth in CCHD. © 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: IGFBP‐3; IGF‐1; brain development; brain volume; congenital heart disease; fetus; magnetic resonance imaging; neonate; trophic factors.
© 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Conflict of interest statement
The PhD position of Maaike Nijman was supported by the Dutch Organization for Health Research and Development (ZonMw); project number 848042002, CRUCIAL trial. The subsidizing party did not play a role in collection, data analysis or writing of this manuscript.
Figures

Similar articles
-
Effects of in utero exposure to multiple metal(loid)s on neonatal birth size and hormones in the growth hormone/insulin-like growth factor axis: A mixture analysis.Environ Pollut. 2025 Oct 1;382:126715. doi: 10.1016/j.envpol.2025.126715. Epub 2025 Jun 20. Environ Pollut. 2025. PMID: 40544944
-
Prenatal interventions for congenital diaphragmatic hernia for improving outcomes.Cochrane Database Syst Rev. 2015 Nov 27;2015(11):CD008925. doi: 10.1002/14651858.CD008925.pub2. Cochrane Database Syst Rev. 2015. PMID: 26611822 Free PMC article.
-
Congenital anomaly and perinatal outcome following blastocyst- vs cleavage-stage embryo transfer: systematic review and network meta-analysis.Ultrasound Obstet Gynecol. 2023 Jan;61(1):12-25. doi: 10.1002/uog.26019. Ultrasound Obstet Gynecol. 2023. PMID: 35751886 Free PMC article.
-
Prevalence of prenatal brain abnormalities in fetuses with congenital heart disease: a systematic review.Ultrasound Obstet Gynecol. 2016 Sep;48(3):296-307. doi: 10.1002/uog.15932. Ultrasound Obstet Gynecol. 2016. PMID: 27062519
-
The Perinatal Committee report: Review of the progress of obstetric healthcare in Japan.J Obstet Gynaecol Res. 2025 Jul;51(7):e16354. doi: 10.1111/jog.16354. J Obstet Gynaecol Res. 2025. PMID: 40690975 Free PMC article. Review.
References
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890‐1900. - PubMed
-
- Liamlahi R, Latal B. Neurodevelopmental outcome of children with congenital heart disease. Handb Clin Neurol. 2019;162:329‐345. - PubMed
-
- Marino BS, Cassedy A, Drotar D, Wray J. The impact of neurodevelopmental and psychosocial outcomes on health‐related quality of life in survivors of congenital heart disease. J Pediatr. 2016;174:11‐22.e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

