Translocation of SIRT6 promotes glycolysis reprogramming to exacerbate diabetic angiopathy
- PMID: 40580755
- PMCID: PMC12269417
- DOI: 10.1016/j.redox.2025.103736
Translocation of SIRT6 promotes glycolysis reprogramming to exacerbate diabetic angiopathy
Abstract
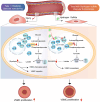
Diabetic angiopathy, a major complication of type 2 diabetes mellitus (T2DM), is driven by vascular dysfunction, metabolic reprogramming, and oxidative stress. NAD+-dependent deacetylase SIRT6, located in the nucleus, is recognized for its role in modulating cardiovascular and metabolic homeostasis through histone deacetylation. However, the functions and mechanisms of accumulation of cytoplasmic SIRT6 in T2DM remain to be elucidated. Herein, a previously unrecognized cytoplasmic role for SIRT6 is identified in promoting pathological glycolysis during diabetic vascular remodeling. Vascular smooth muscle cell (VSMC) proliferation is observed, which is correlated with protein deacetylation, especially SIRT6, which translocated to the cytoplasm mediated by Importin 13 (IPO13). Furthermore, the accumulation of cytoplasmic SIRT6 facilitates its interaction with enolase 3 (ENO3), a newly discovered downstream target. This interaction promotes ENO3 deacetylation, enhances downstream phosphoenolpyruvic acid (PEP) levels, and thereby drives glycolysis reprogramming, ultimately leading to the pathological changes associated with diabetic angiopathy. Notably, exogenous hydrogen sulfide (H2S) restores S-sulfhydration of SIRT6 at cysteine 141, counteracting the SIRT6-ENO3 interaction, suppressing glycolysis, and mitigating VSMC hyperproliferation. This study provides novel insights into the SIRT6-ENO3 pathway through regulating vascular glycolysis reprogramming, highlighting the therapeutic potential of targeting SIRT6 in the management of diabetic angiopathy.
Keywords: Cell proliferation; Glycolysis; Hydrogen sulfide (H(2)S); Sirtuin 6 (SIRT6); Type 2 diabetes (T2D).
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures








References
-
- Zucatti K.P., Teixeira P.P., Wayerbacher L.F., Piccoli G.F., Correia P.E., Fonseca N.K.O., et al. Long-term effect of lifestyle interventions on the cardiovascular and all-cause mortality of subjects with prediabetes and type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2022;45:2787–2795. doi: 10.2337/dc22-0642. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

