Protection from killed whole-cell cholera vaccines: a systematic review and meta-analysis
- PMID: 40580988
- PMCID: PMC12208782
- DOI: 10.1016/S2214-109X(25)00107-X
Protection from killed whole-cell cholera vaccines: a systematic review and meta-analysis
Abstract
Background: Killed whole-cell oral cholera vaccines (kOCVs) are a standard prevention and control measure in cholera-endemic areas and during outbreaks and humanitarian emergencies. New evidence has emerged and the ways in which the vaccines are used have changed. We aimed to provide an updated synthesis of evidence on protection conferred by kOCV.
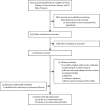
Methods: In this systematic review and meta-analysis, we used the same search procedure as a previous systematic review to identify randomised clinical trials (RCTs) and observational studies that reported estimates of protection conferred by kOCVs against medically attended, confirmed cholera. Eligible studies in English, French, Spanish, or Chinese published up until March 8, 2024, including those identified in the previous review, were included. Data on efficacy and effectiveness were extracted, as were the number of doses, duration of follow-up, and age group. Efficacy and effectiveness estimates were summarised separately using random-effect models to estimate protection by time since vaccination; meta-regression models were used to estimate protection, by dose, as a function of time since vaccination. This updated study is registered along with the original review with PROSPERO (CRD42016048232).
Findings: We identified 8205 records published online up until March 8, 2024, including 6224 articles from the previous review and 1981 articles from our new search (after Jan 1, 2016). Of these, 53 were eligible for full-text review. Five RCTs and ten observational studies from 23 publications were included. Average two-dose efficacy 12 months after vaccination was 55% (95% CI 46-62), declining to 44% (25-59) 48 months after vaccination. Average two-dose effectiveness was 69% (58-78) 12 months after vaccination, declining to 47% (9-70) 48 months after vaccination. Only one RCT assessed one-dose efficacy and found sustained protection for 24 months (57% [42-69]) among those 5 years and older with no significant protection in younger children. Average one-dose effectiveness 12 months after vaccination was 60% (51-68) and after 24 months was 47% (34-58). Using age group-specific meta-analysis, we found that average two-dose efficacy in children younger than 5 years was half that of older individuals.
Interpretation: Two doses of kOCV provide protection against medically attended cholera for at least 4 years after vaccination. One dose of kOCV provides protection for at least 2 years after vaccination, but wanes faster than that of two doses. Children younger than 5 years are less protected by kOCVs than those aged 5 years and older, regardless of the number of doses received.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention. The following authors are members of working groups of the Global Task Force for Cholera Control (GTFCC) including the Oral Cholera Vaccine Working Group: AT, DG, FJL, SK, GB, FQ, LCI, VM, MB, LB (chairperson), and ASA. The following authors are members of the Cholera Surveillance Working Group: ECL, ASA, and EBM. MB and VM are cholera focal points for the Global Task Force for Cholera Control. DG is on the steering committee for the GTFCC. Within the International Coordinating Group on vaccine provision, which makes decisions related to the global oral cholera vaccine stockpile for outbreaks and emergencies, DG has represented Médecins Sans Frontières, MB and VM have represented WHO, and FJL has represented Gavi, the Vaccine Alliance. FQ was a former member of the WHO Strategic Advisory Group of Experts (SAGE), and FJL was a member of the SAGE Working Group on cholera vaccines that was convened before the 2017 WHO position paper was developed. FL is an employee of Gavi, which supports countries with procurement and delivery of oral cholera vaccines, and serves as Gavi representative in the Steering Committee of the GTFCC. ASA is a member of the Gavi Independent Review Committee. All other authors declare no competing interests.
Figures


References
-
- Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986;2:124–127. - PubMed
-
- Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. - PubMed
-
- Qadri F, Ali M, Chowdhury F, et al. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet. 2015;386:1362–1371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

