Systemic metabolic changes in acute and chronic lymphocytic choriomeningitis virus infection
- PMID: 40581045
- PMCID: PMC12274302
- DOI: 10.1016/j.molmet.2025.102194
Systemic metabolic changes in acute and chronic lymphocytic choriomeningitis virus infection
Erratum in
-
Corrigendum to 'Systemic metabolic changes in acute and chronic lymphocytic choriomeningitis virus infection' [Mol Metab Volume 99 (2025) Article 102194].Mol Metab. 2025 Sep;99:102217. doi: 10.1016/j.molmet.2025.102217. Epub 2025 Jul 31. Mol Metab. 2025. PMID: 40749740 Free PMC article. No abstract available.
Abstract
Objective: Viral infection of cells leads to metabolic changes, but how viral infection changes whole-body and tissue metabolism in vivo has not been comprehensively studied. In particular, it is unknown how metabolism might be differentially affected by an acute infection that the immune system can successfully clear compared to a chronic persistent infection.
Methods: Here we used metabolomics and isotope tracing to identify metabolic changes in mice infected with acute or chronic forms of lymphocytic choriomeningitis virus (LCMV) for three or eight days.
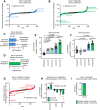
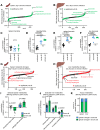
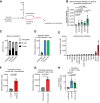
Results: Both types of infection alter metabolite levels in blood and tissues, including itaconate and thymidine. However, we observed more dramatic metabolite changes in the blood and tissues of mice with persisting LCMV infection compared to those infected with the acute viral strain. Isotope tracing revealed that the contribution of both glucose and glutamine to the tricarboxylic acid (TCA) cycle increase in the spleen, liver, and kidneys of mice infected with chronic LCMV, while acute LCMV only increases the contribution of glutamine to the TCA cycle in the spleen. We found that whole-body turnover of both glutamine and thymidine increase during acute and chronic infection, whereas whole-body glucose turnover surprisingly does not change. Activated T cells in vitro produce thymidine and virus-specific T cells ex vivo have increased thymidine levels, nominating T lymphocytes as the source of thymidine in LCMV infection.
Conclusions: In sum, we provide comprehensive measurements of whole-body and tissue metabolism in acute and chronic viral infection, and identify altered thymidine metabolism as a marker of viral infection.
Keywords: Immunometabolism; Isotope tracing; Metabolomics; Tissue metabolism; Whole-body metabolism.
Copyright © 2025 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Joshua D. Rabinowitz reports a relationship with Bantam Pharmaceutical LLC that includes: consulting or advisory and equity or stocks. Joshua D. Rabinowitz reports a relationship with Pfizer Inc that includes: consulting or advisory. Joshua D. Rabinowitz reports a relationship with Third Rock Ventures LLC that includes: consulting or advisory. Joshua D. Rabinowitz reports a relationship with Empress Therapeutics, Inc. that includes: equity or stocks. Joshua D. Rabinowitz is an advisor and stockholder in Colorado Research Partners, L.E.A.F. Pharmaceuticals, Barer Institute, and Rafael Pharmaceuticals; a founder, director, and stockholder of Farber Partners, Serien Therapeutics, and Sofro Pharmaceuticals; and a director of the Princeton University-PKU Shenzhen collaboration. - JDR. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Buchmeier M.J., Welsh R.M., Dutko F.J., Oldstone M.B.A. In: Dixon F.J., Kunkel H.G., editors. vol. 30. Academic Press; 1980. The virology and immunobiology of lymphocytic choriomeningitis virus infection; pp. 275–331. (Advances in immunology). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

