Early detection of Alzheimer's disease using small RNAs. Results from the EPAD cohort
- PMID: 40581531
- PMCID: PMC12413721
- DOI: 10.1016/j.tjpad.2025.100257
Early detection of Alzheimer's disease using small RNAs. Results from the EPAD cohort
Abstract
Background: Alzheimer's disease (AD) is the most common form of dementia, and early diagnosis is crucial to enable effective interventions. Currently, Alzheimer's disease is diagnosed through cognitive assessments, brain imaging and fluid biomarkers focused on determining amyloid (A) and, tau (T) protein levels as well as neurodegeneration (N) in the AT(N) framework. Prognostic biomarkers for predicting cognitive decline within the amyloid positive (Aβ+) individuals would further strengthen the framework.
Objectives: This study evaluated small RNAs as novel auxiliary biomarkers, independent of the AT(N) framework, either alone or in combination with established protein markers, for detecting the earliest cognitive decline in AD.
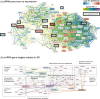
Design: The European Prevention of Alzheimer's Disease (EPAD) clinical trial platform is a prospective, multi-center study designed to investigate biomarkers for preclinical and prodromal AD.
Setting: Peripheral whole blood RNA sequencing was performed on participants across Europe with no cognitive impairment or very mild cognitive impairment (MCI), stratified by cerebrospinal fluid amyloid levels.
Participants: 1,913 participants, 50 years or older and free of dementia diagnosis at enrollment, were analyzed.
Intervention: (if any) Not applicable.
Measurements: Ultra-deep small RNA sequencing was performed on whole blood samples using a refined blocking protocol to eliminate highly abundant erythroid small RNAs, and thereby to open sequencing bandwidth for the discovery of less abundant biomarker RNAs. Biomarker RNAs were deconvolved into plasma or blood cell origin and analyzed for functional relevance. We define high and low amyloid groups based on a cutoff on the p-tau181/Aβ1-42 ratio as determined from cerebrospinal fluid.
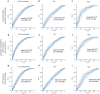
Results: We identified a combination of small RNAs that predicted early cognitive decline (Clinical Dementia Rating of 0.5) with an area under the receiver-operator curve of ∼0.7. Notably, when focusing on individuals with cognitive decline and high amyloid burden (Aβ+), the predictive accuracy improved to an AUC of 0.77. This performance could be extended to the entire cohort when combining blood RNA and CSF amyloid markers (AUC 0.76). We conducted bioinformatic analyses to interrogate the likely functional relevance of these small RNAs, uncovering several links to dementia-relevant pathways, including neuronal, cardiovascular, and inflammatory activities. Our findings also suggest that small nucleolar RNAs warrant further investigation as potential disease-relevant markers, in addition to microRNAs.
Conclusions: Integrating small RNA biomarkers with protein-based assays offers preliminary evidence for stratifying MCI, particularly within the amyloid positive continuum. Small nucleolar RNAs and microRNAs warrant further exploration as complementary diagnostic tools, and their use may enable more precise and effective interventions.
Keywords: AT(N) framework; Blood RNA biomarker; Early Onset Alzheimer’s Disease (EOAD); European Prevention for Alzheimer’s Dementia (EPAD); Machine learning; Mild Cognitive Impairment (MCI); miRNA; small nucleolar RNA (snoRNA).
Copyright © 2025. Published by Elsevier Masson SAS.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tobias Sikosek (TS), Jagoda Mika (JM), Mustafa Kahraman (MK), Julia Jehn (JJ), Alberto Daniel-Moreno (ADM), Jessika Ceiler (JC), Jasmin Skottke (JS), Marta Sanchez-Delgado (MSD), Patrick Neubert (PN), Christina Rudolf (CRu), Kaja Tikk (KT), Rastislav Horos (RH), and Bruno Steinkraus (BS) are current or previous employees of Hummingbird Diagnostics. Marco Heuvelman (MH) and Maurice Frank (MF) are paid consultants for Hummingbird Diagnostics. Jeffrey L. Cummings (JLC) has provided consultation to Acadia, Acumen, ALZpath, Annovis, Aprinoia, Artery, Axsome, Biogen, Biohaven, BioXcel, Bristol-Myers Squibb, Cervomed, Eisai, Fosun, GAP Foundation, Green Valley, Hummingbird Diagnostics, IGC, Janssen, Kinoxis, Lighthouse, Lilly, Lundbeck, LSP/eqt, Mangrove Therapeutics, Merck, MoCA Cognition, New Amsterdam, Novo Nordisk, NSC Therapeutics, Optoceutics, Otsuka, Oxford Brain Diagnostics, Praxis, Prothena, ReMYND, Roche, Scottish Brain Sciences, Signant Health, Simcere, Sinaptica, T-Neuro, TrueBinding, and Vaxxinity pharmaceutical, assessment, and investment companies. JLC is also a member of the editorial board of the Journal of Prevention of Alzheimer’s Disease. Craig Ritchie (CR) is the Founder, CEO, and majority shareholder of Scottish Brain Sciences, which has received compensation for study-related activities from AC Immune SA. CR has received consulting fees from Biogen, Eisai, MSD, Actinogen, Roche, Eli Lilly, and Novo Nordisk, as well as honoraria for lectures from Roche, Eisai, and Eli Lilly. CR is also a member of the advisory board for Novo Nordisk. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Nandi A., Counts N., Chen S., Seligman B., Tortorice D., Vigo D., et al. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: a value of statistical life approach. EClin Med. 2022;51 doi: 10.1016/j.eclinm.2022.101580. - DOI - PMC - PubMed
-
- Schindler S.E., Galasko D., Pereira A.C., Rabinovici G.D., Salloway S., Suárez-Calvet M., et al. Acceptable performance of blood biomarker tests of amyloid pathology — Recommendations from the global CEO initiative on Alzheimer’s disease. Nature Rev Neurol. 2024;20:426–439. doi: 10.1038/s41582-024-00977-5. Nature Research. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

