Genetically-engineered Salmonella typhimurium expressing FGF21 promotes neurological recovery in ischemic stroke via FGFR1/AMPK/mTOR pathway
- PMID: 40581646
- PMCID: PMC12205506
- DOI: 10.1186/s12974-025-03498-0
Genetically-engineered Salmonella typhimurium expressing FGF21 promotes neurological recovery in ischemic stroke via FGFR1/AMPK/mTOR pathway
Abstract
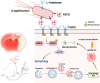
Background: Ischemic stroke (IS) remains a leading cause of mortality and disability, with limited therapeutic options due to poor drug delivery to ischemic lesions. To address this challenge, an engineered Salmonella based therapeutic method for targeted drug delivery and long-term treatment is herein designed to mitigate ischemic damage.
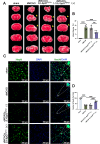
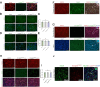
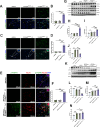
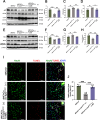
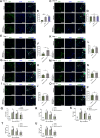
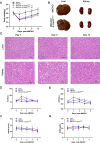
Methods: We engineered an attenuated luminescent Salmonella typhimurium (S.t -ΔpG) strain with an L-arabinose-inducible pBAD system to secrete bioactive FGF21. C57BL/6 mice were used to to measure neuron apoptosis and the activity of immune cells following IS induction plus S.t-ΔpG injection. Bioluminescence imaging was applied for bacterial colonization. ELISA and glucose uptake assays were performed to detect FGF21 secretion and the bioactivity. Neurological tests, TTC staining, and TUNEL labeling were used to assess the therapeutic effects of barterially secreted FGF21. Immunofluorescence assay of FGF21/FGFR1 dominant pathway was explored to investigate neuroprotective mechanism, while IBA-1 staining, CD3/CD68 immunostaining, cytokine profiling, and hepatorenal histopathology were detected to evaluate biosecurity.
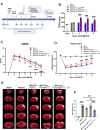
Results: S.t-ΔpGFGF21 selectively colonized peri-infarct regions and secreted functional FGF21, reducing neurologic deficits (48%) and infarct volume (46%) versus controls (p < 0.01). Mechanistically, immunofluorescence demonstrated that bacterially secreted FGF21 activated neuronal FGFR1/AMPK/mTOR pathway to enhance autophagy, whereas autophagy inhibition abolished its neuroprotection. Further, bacterial exclusion from neuron was validated via MAP2/NeuN plus Salmonella co-staining in primary neuron cells and brain tissue. Critically, CD3/CD68 immunostaining, serum cytokine profiling, and hepatorenal histopathology confirmed the long-term biosafety of this approach.
Conclusion: Our study presents a novel, Salmonella - based platform for targeted and sustained FGF21 delivery, offering a promising therapeutic strategy for ischemic stroke with robust efficacy and minimal systemic toxicity.
Keywords: Salmonella typhimurium; FGF21; FGFR1/AMPK/mTOR pathway; Hepatorenal histopathology; Ischemic stroke; Neurologic deficit.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University. Consent for publication: All authors approved this manuscript and provided consent for publication. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Development of FGF21 Mutant with Potent Cardioprotective Effects in T2D Mice via FGFR1-AMPK-Mediated Inhibition of Oxidative Stress.Int J Mol Sci. 2025 Jul 9;26(14):6577. doi: 10.3390/ijms26146577. Int J Mol Sci. 2025. PMID: 40724827 Free PMC article.
-
Saponins from Panax japonicus Enhance Lipolysis via Acting on FGF21-β-Klotho/FGFR1 in Obese Mice.J Agric Food Chem. 2025 Jun 25;73(25):15624-15636. doi: 10.1021/acs.jafc.5c01012. Epub 2025 Jun 13. J Agric Food Chem. 2025. PMID: 40509879
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
Cited by
-
New insights into acute ischemic stroke from the perspective of spatial omics.Theranostics. 2025 Jul 11;15(15):7902-7924. doi: 10.7150/thno.113396. eCollection 2025. Theranostics. 2025. PMID: 40756344 Free PMC article. Review.
References
-
- Sun H, Ma B, Jin C, Li Z, Song X, Bu Y, et al. Global, regional, and National burdens of stroke in children and adolescents from 1990 to 2019: A Population-Based study. Stroke. 2024;55(6):1543–53. - PubMed
-
- Al-Ajlan FS, Alkhiri A, Alamri AF, Alghamdi BA, Almaghrabi AA, Alharbi AR, et al. Golden hour intravenous thrombolysis for acute ischemic stroke: A systematic review and Meta-Analysis. Ann Neurol. 2024;96(3):582–90. - PubMed
-
- Warach SJ, David G. Sherman lecture: improving stroke diagnosis and Treatment-A journey toward the end of time. Stroke. 2024;55(10):2567–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

