Parvalbumin interneurons gate amygdala excitability and response to chronic stress via kainate receptor-driven tonic GABAB receptor-mediated inhibition
- PMID: 40581655
- PMCID: PMC12532722
- DOI: 10.1038/s41380-025-03093-y
Parvalbumin interneurons gate amygdala excitability and response to chronic stress via kainate receptor-driven tonic GABAB receptor-mediated inhibition
Abstract
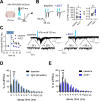
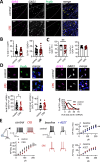
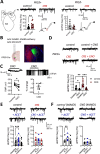
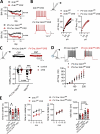
Amygdala hyperexcitability is a hallmark of stress-induced anxiety disorders. Stress-associated changes in both principal neurons and interneurons contribute to the increased excitability, but how exactly these mechanisms interact to regulate the function of behaviorally relevant circuits in the amygdala remains unclear. Here, we show that GluK1 subunit-containing kainate receptors in parvalbumin (PV) interneurons maintain high GABA release and control excitability of lateral amygdala (LA) principal neurons via tonic GABAB-receptor-mediated inhibition. Downregulation of GluK1 expression in PV interneurons after chronic restraint stress (CRS) releases the tonic inhibition and increases excitability of LA principal neurons. Stress-induced LA hyperexcitability was associated with increased glutamatergic transmission to central amygdala PKCδ-expressing neurons, implicated in fear generalization. Consistent with significance in anxiogenesis, absence of GluK1-GABAB regulation confers resilience against CRS-induced LA hyperexcitability and anxiety-like behavior. Our data reveal a unique novel mechanism involving an interplay between glutamatergic and GABAergic systems in the regulation of amygdala excitability in response to chronic stress.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were conducted in accordance with the University of Helsinki Animal Welfare Guidelines and approved by the National Animal Experiment Board of Finland (license numbers: KEK-17-019, KEK22-010, ESAVI/29384/2019, and ESAVI/31984/2022).
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

