Explainable, federated deep learning model predicts disease progression risk of cutaneous squamous cell carcinoma
- PMID: 40581685
- PMCID: PMC12206231
- DOI: 10.1038/s41698-025-00997-4
Explainable, federated deep learning model predicts disease progression risk of cutaneous squamous cell carcinoma
Abstract
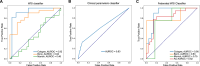
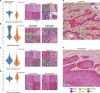
Predicting cancer patient disease progression is a key step towards personalized medicine and secondary prevention. Risk stratification systems based on clinico-pathological criteria aim to identify high-risk patients, but accurate predictions remain challenging. Deep learning models present new opportunities for patient risk prediction, yet their interpretability has been largely unexplored. We developed a transformer-based approach for predicting progression of cutaneous squamous cell carcinoma (cSCC) patients based on diagnostic histopathology tumor slides. Our initial model showed AUROC = 0.92 on a held-out test set, with average AUROC of 0.65 on external validation cohorts. To further increase generalizability and reduce potential privacy concerns, we trained the model in a federated manner across three clinical centers, reaching AUROC = 0.82 across all cohorts, with image-based risk scores achieving hazard ratios up to 7.42 (p < 0.01) in multivariable analyses. Through interpretability analysis, we identified spatial and morphological features predictive of progression, suggesting that tumor boundary information and tissue heterogeneity characterize progressive cSCCs. Trained exclusively on routine diagnostic slides and offering biological insights, our model can improve secondary prevention and understanding of cSCC while enabling deployment across clinical centers without administrative overheads or privacy concerns.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The Authors declare no Competing Non-Financial Interests but the following Competing Financial Interests: D.N. received financial support (speaker’s honoraria, advisory boards, travel expense reimbursements or grants) from Abbvie, Almirall, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers-Squib, GlaxoSmithKline, Incyte, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Lilly, L’Oreal/Cerave, MSD, Novartis, Pfizer, Regeneron and UCB Pharma. J.B. received research funding from Bayer and travel expenses from Merck KG and Bicycle Therapeutics outside the presented work. K.D. received financial support (speaker’s honoraria, advisory boards, travel expense reimbursements or grants) from Abbvie, Bristol-Myers-Squib, Novartis, and Pierre-Fabre.
Figures



References
-
- Keim, U. et al. Incidence, mortality and trends of cutaneous squamous cell carcinoma in Germany, the Netherlands, and Scotland. Eur. J. Cancer Oxf. Engl. 1990183, 60–68 (2023). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

