29-mRNA host response signatures for classification of bacterial infection, viral infection and disease progression in COVID-19 pneumonia: a post hoc analysis of the SAVE-MORE randomized clinical trial
- PMID: 40583075
- PMCID: PMC12206681
- DOI: 10.1186/s40635-025-00777-1
29-mRNA host response signatures for classification of bacterial infection, viral infection and disease progression in COVID-19 pneumonia: a post hoc analysis of the SAVE-MORE randomized clinical trial
Abstract
Background: Biomarkers based on host response signatures are currently under development for the critically ill. We applied a 29-mRNA classifier for the diagnosis and prognosis of suspected acute infection and sepsis (TriVerity™, Inflammatix Inc.) in patients hospitalized with COVID-19.
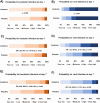
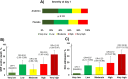
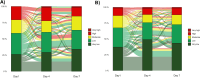
Methods: We applied three scores from locked classifiers (IMX-BVN-4 and IMX-SEV-4) from the 29-mRNA TriVerity™ blood test in participants of the SAVE-MORE randomized clinical trial (ClinicalTrials.gov NCT04680949) at baseline and days 4 and 7 of treatment, to classify bacterial infection, viral infection and decompensation. Participants were adults hospitalized with confirmed COVID-19 pneumonia and plasma soluble urokinase plasminogen activator receptor (suPAR) levels of ≥ 6 ng/ml, randomized to placebo or anakinra treatment.
Results: A total of 471 patients were studied. At baseline nearly 90% had a Very Low or Low IMX-BVN-4 Bacterial Score and Moderate, High or Very High IMX-BVN-4 Viral Score. Anakinra treatment had an effect on the expression of genes indicating IMX-SEV-4 High or Very High scores after a 7 day treatment compared to baseline (12.9% of anakinra-treated patients continued being classified as high severity vs 20.4% of placebo-treated patients, p 0.046).
Conclusions: The classifiers were well tested in COVID-19 pneumonia and may become a useful tool for hospitalized patients.
Keywords: Anakinra; Bacterial infection; COVID-19; Diagnostics; Gene expression; Host response; Mortality; Secondary infection; Severity; Viral infection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The SAVE-MORE trial (NCT04680949) was approved by the National Ethics Committee of Greece (approval 161/20) and by the Ethics Committee of the National Institute for Infectious Diseases Lazzaro Spallanzani, IRCCS, in Rome (1 February 2021). All patients or their legal representatives provided written informed consent before enrollment. Consent for publication: Not applicable. Competing interests: TS is an employee of and shareholder in Inflammatix, Inc. OL and PK are shareholders in Inflammatix, Inc. EJG-B reports honoraria from Abbott Products Operations, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi and Xbiotech Inc; independent educational grants from Abbott Products Operations, bioMérieux Inc, Johnson & Johnson, MSD, UCB, Swedish Orphan Biovitrum AB; and funding from the Horizon 2020 European Grants ImmunoSep and RISCinCOVID and the Horizon Health grants EPIC-CROWN-2, POINT and Homi-Lung (granted to the Hellenic Institute for the Study of Sepsis). The other authors declare no competing interests.
Figures
References
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801 - PMC - PubMed
-
- Kostaki A, Wacker JW, Safarika A, Solomonidi N, Katsaros K, Giannikopoulos G, Koutelidakis IM, Hogan CA, Uhle F, Liesenfeld O, Sweeney TE, Giamarellos-Bourboulis EJ (2022) A 29-mRNA host response whole-blood signature improves prediction of 28 day mortality and 7 day intensive care unit care in adults presenting to the emergency department with suspected acute infection and/or sepsis. Shock 58:224 - PMC - PubMed
-
- Thair S, Mewes C, Hinz J, Bergmann I, Büttner B, Sehmisch S, Meissner K, Quintel M, Sweeney TE, Khatri P, Mansur A (2021) Gene expression-based diagnosis of infections in critically Ill patients-prospective validation of the SepsisMetaScore in a longitudinal severe trauma cohort. Crit Care Med 49:e751 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

