Tryptophan Metabolite Indole-3-Aldehyde Induces AhR and c-MYC Degradation to Promote Tumor Immunogenicity
- PMID: 40583248
- PMCID: PMC12463101
- DOI: 10.1002/advs.202409533
Tryptophan Metabolite Indole-3-Aldehyde Induces AhR and c-MYC Degradation to Promote Tumor Immunogenicity
Abstract
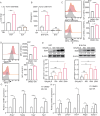
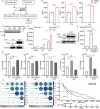
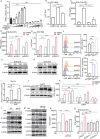
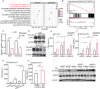
The role of tryptophan (Trp) and its metabolites in immune regulation is well-established, however, whether and how they regulate immunogenicity of tumor cells is not completely understood. In this study, a range of Trp metabolites are evaluated for their potential to modulate tumor immunogenicity using a co-culture assay with tumor cells and T cells. Indole-3-aldehyde (I3A) is identified as an indole derivative that significantly enhances tumor immunogenicity both in vitro and in vivo. This enhancement is attributed to the upregulation of antigen presentation and immunogenic molecules on tumor cells by I3A, thereby promoting their immunogenicity. Mechanistically, I3A induces the activation and degradation of Aryl hydrocarbon receptor (AhR), leading to increased expression of MHC-I molecules on tumor cell surfaces. Meantime, I3A induces rapid degradation of c-MYC in tumor cells and further enhances T cell activation. In mouse melanoma and lymphoma models, I3A demonstrates immune-dependent antitumor effects and enhances the efficacy of adoptive OT-I T cell therapy. Moreover, overexpression of the Trp metabolic enzyme interleukin-4-induced gene-1 (IL4I1) in tumor cells increases the intracellular level of I3A and enhances tumor immunogenicity. In summary, I3A is identified as a tumor immunogenicity inducer, which holds the potential to enhance antitumor immunotherapy efficacy.
Keywords: AhR; c‐Myc; indole‐3‐aldehyde; tryptophan metabolism; tumor immunogenicity.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
