Esophageal endoscopic findings after pulmonary vein and posterior wall isolation using pulsed field ablation: results from the Eso-PFA study
- PMID: 40583630
- PMCID: PMC12342907
- DOI: 10.1093/europace/euaf133
Esophageal endoscopic findings after pulmonary vein and posterior wall isolation using pulsed field ablation: results from the Eso-PFA study
Abstract
Aims: Substrate modification, including left atrial posterior wall isolation (LAPWI), may be performed in AF patients undergoing catheter ablation. Pulsed field ablation (PFA) may protect adjacent structures like the esophagus. However, data on esophageal safety following PFA-guided LAPWI are limited. The aim is to evaluate esophageal safety during post-procedural esophagogastroduodenoscopy (EGD) and follow-up after PFA-guided LAPWI in patients with AF.
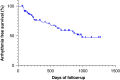
Methods and results: This prospective observational study included consecutive AF patients who underwent PFA-guided LAPWI and post-procedural EGD (the day after PFA), with follow-up for long-term safety. In total, 106 consecutive patients (94% persistent AF, 66 ± 14 years, 70% male, 2 ± 1 prior ablation procedures) were included. The total median procedure time was 78 [interquartile range (IQR): 49-111] min, and a mean of 50 ± 19 and 21 ± 9 PFA applications were delivered per patient and on the LAPW, respectively. One suspected transient ischaemic attack and three minor complications occurred. No thermal, ablation-related esophageal lesions were observed in any patient during EGD. Non-ablation-related incidental gastrointestinal findings were detected in 70% of patients. During a median follow-up of 606 days [IQR: 212-922], no additional esophageal adverse events were reported. Atrial arrhythmia recurrences occurred in 34% (36/106) patients (including antiarrhythmic drugs). Left atrial posterior wall isolation durability was 78% (11/14).
Conclusion: In this real-world cohort, PFA-guided LAPWI was safe for the esophagus, with no thermal injury observed in post-procedural endoscopy. These results further support PFA as a promising technology for AF ablation with a favourable esophageal safety profile. The role of post-ablation proton pump inhibitors in a population where incidental gastrointestinal findings were common needs further exploration.
Keywords: Atrial fibrillation; Endoscopy; Persistent atrial fibrillation; Posterior wall isolation; Pulmonary vein isolation; Pulsed field ablation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: M.A.G.: speaker’s honoraria, educational grants, fellowship, consultation fees: Boston Scientific/Farapulse Inc., Medtronic, Biosense Webster/J&J Medtech, Abbott, Biotronik, Zoll, Bristol Myers Squibb. M.M.: none regarding this work. U.-F.P.: no CoI with regard to the topic of the manuscript. S.Maasberg: none regarding this work. S.Matuschka: none regarding this work; J.H.: none regarding this work. J.D.: none regarding this work. R.W.: none regarding this work. T.H.: none regarding this work. A.S.: consultation fees/educational grants Boston Scientific. B.D.: none regarding this work. P.S.: reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott Medical, Boston Scientific, and Becton-Dickenson. P.S. reports serving on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx, and Pacemate. S.W. reports receiving grants and personal fees from Abbott, Boston Scientific, and Medtronic, and personal fees from Bristol Myers Squibb, Johnson & Johnson, and Farapulse Inc.
Figures
References
-
- Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2024;45:3314–414. - PubMed
-
- Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. - PMC - PubMed
-
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. - PubMed
-
- Kistler PM, Chieng D, Sugumar H, Ling L-H, Segan L, Azzopardi S et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. - PMC - PubMed
-
- Ariyaratnam JP, Middeldorp ME, Brooks AG, Thomas G, Kadhim K, Mahajan R et al. Coronary Sinus isolation for high-burden atrial fibrillation: a randomized clinical trial. JACC Clin Electrophysiol 2025;11:1–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical