Uncemented hip arthroplasty and denosumab: increased postoperative dipeptide concentrations and identification of potential new bone turnover biomarkers
- PMID: 40584156
- PMCID: PMC12202150
- DOI: 10.1093/jbmrpl/ziaf091
Uncemented hip arthroplasty and denosumab: increased postoperative dipeptide concentrations and identification of potential new bone turnover biomarkers
Abstract
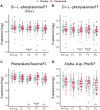
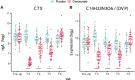
Denosumab is a potent antagonist of RANKL and is widely used to treat severe postmenopausal osteoporosis. Using high-resolution mass spectrometry (HRMS), we aimed to identify molecular mediators associated with the rapid reactivation of osteoclasts following discontinuation of denosumab. In a previously reported randomized controlled trial, 64 patients undergoing uncemented total hip arthroplasty were randomized to receive 2 doses of 60 mg denosumab or placebo, administered 1-3 d and 6 mo postoperatively. Serum samples were analyzed using untargeted HRMS coupled with liquid chromatography, and bone turnover markers were assessed. Data were evaluated using linear mixed-effects models and machine learning techniques. After surgery, 83 metabolite features showed significant concentration changes (p < .0001). Denosumab-treated patients exhibited increased levels of the dipeptides di-L-phenylalanine, phenylalanylleucine, and alpha-Asp-Phe, and decreased levels of fibrinopeptide A and related peptides 24 mo after surgery. The oxidized peptide AP(Ox)GDRGEP(Ox)GPP(Ox)GP, derived from the collagen type I alpha 1 chain (COL1A1) and referred to as COL1A1-OxP, showed a strong correlation with the bone formation marker procollagen type 1 amino-terminal propeptide (P1NP) (p = 4.4E-83). Similarly, the tripeptide DL-alpha-aspartyl-DL-valyl-DL-proline (DVP) correlated highly with the bone resorption marker carboxy-terminal telopeptide of type 1 collagen (CTX) (p = 1.1E-222). P1NP and CTX levels were suppressed at 3, 6, and 12 mo postoperatively but exceeded baseline levels by 24 mo. Global metabolic shifts were observed postoperatively, with distinct profiles between treatment groups. The observed increase in specific dipeptides may reflect mechanisms contributing to rebound bone loss following denosumab withdrawal. Fibrinopeptide A and its analogs may play a protective role, while COL1A1-OxP and DVP represent potential new markers of bone turnover. These findings suggest metabolomics-based biomarkers could aid clinical decision-making by allowing earlier detection of rebound effects and guiding individualized treatment strategies after denosumab therapy. Clinical trial registration number: ClinicalTrials.gov, NCT01630941 (URL: https://clinicaltrials.gov/); European Union Clinical Trials Register (EU CTR), EudraCT No. 2011-001481-18 (https://www.clinicaltrialsregister.eu/).
Keywords: bone turnover biomarkers; denosumab; dipeptides; high-resolution mass spectrometry; metabolomics; osteoclast activity; periprosthetic bone loss; rebound bone loss; total hip arthroplasty.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
N.P.H. has received institutional support, lecturer’s fees, or honoraria from Waldemar Link, Hamburg, Germany; Zimmer Biomet, Warsaw, IN, USA; DePuy Synthes, Stockholm, Sweden; Heraeus Medical, Wehrheim, Germany; and has a license agreement with Waldemar Link, Hamburg, Germany. On behalf of us and the remaining co-authors, we declare that we have no conflicts of interest and nothing to disclose.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

