Comparative evaluation of four new immunoassays and LC-MS/MS for the measurement of urinary free cortisol in Cushing's syndrome diagnosis
- PMID: 40584217
- PMCID: PMC12205834
- DOI: 10.1016/j.plabm.2025.e00484
Comparative evaluation of four new immunoassays and LC-MS/MS for the measurement of urinary free cortisol in Cushing's syndrome diagnosis
Abstract
Objectives: Twenty-four-hour urinary free cortisol (UFC) measurement is the initial diagnostic test for Cushing's syndrome (CS). We compared UFC determination by four new immunoassays using Autobio A6200, Mindray CL-1200i, Snibe MAGLUMI X8 and Roche 8000 e801 with liquid chromatography-tandem mass spectrometry (LC-MS/MS). Additionally, we evaluated the value of 24-h UFC measured by four direct immunoassays for diagnosing CS.
Methods: Residual 24-hr urine samples of 94 CS and 243 non-CS patients collected from previous cohort were used. A laboratory-developed LC-MS/MS method was used as reference. UFC was measured by immunoassays using Autobio, Mindray, Snibe and Roche platforms. Method was compared using Passing-Bablok regression and Bland-Altman plot analyses. Cut-off values for each assay and corresponding sensitivities and specificities were calculated by ROC analysis.
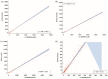
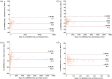
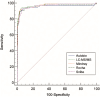
Results: All four immunoassays showed strong correlations with LC-MS/MS (Spearman coefficient r = 0.950, 0.998, 0.967, and 0.951, respectively). All immunoassays showed proportionally positive bias. The areas under the curve were 0.953 for Autobio, 0.969 for Mindray, 0.963 for Snibe, and 0.958 for Roche. The cut-off values varied from 178.5 to 272.0 nmol/24 h). Assay sensitivity and specificity ranged from 89.66 % to 93.10 % and from 93.33 % to 96.67 %, respectively.
Conclusions: Four newly available direct immunoassays for measuring UFC show good analytical consistency compared to LC-MS/MS. The elimination of organic solvent extraction simplifies workflows while maintaining high diagnostic accuracy. Additionally, they exhibited similarly high diagnostic accuracy for CS identification. Future multi-center studies are needed to validate our findings and establish method-specific UFC cut-off values to enhance clinical utility.
Keywords: Cushing's syndrome; Immunoassay; Liquid chromatography-tandem mass spectrometry; Method comparison; Urinary free cortisol.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Comparison of Direct and Extraction Immunoassay Methods With Liquid Chromatography-Tandem Mass Spectrometry Measurement of Urinary Free Cortisol for the Diagnosis of Cushing's Syndrome.Ann Lab Med. 2024 Jan 1;44(1):29-37. doi: 10.3343/alm.2024.44.1.29. Epub 2023 Sep 4. Ann Lab Med. 2024. PMID: 37665283 Free PMC article.
-
Performance of LC-MS/MS and immunoassay based 24-h urine free cortisol in the diagnosis of Cushing's syndrome.J Steroid Biochem Mol Biol. 2019 Jun;190:193-197. doi: 10.1016/j.jsbmb.2019.04.004. Epub 2019 Apr 5. J Steroid Biochem Mol Biol. 2019. PMID: 30959155
-
Matching-Adjusted Indirect Comparison of Osilodrostat Versus Metyrapone for the Treatment of Cushing's Syndrome.Adv Ther. 2025 Jul;42(7):3472-3485. doi: 10.1007/s12325-025-03229-0. Epub 2025 May 29. Adv Ther. 2025. PMID: 40439958
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
References
-
- Pivonello R., Isidori A.M., De Martino M.C., Newell-Price J., Biller B.M., Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. - PubMed
-
- Webb S.M., Valassi E. Morbidity of cushing's syndrome and impact of treatment. Endocrinol Metab. Clin. N. Am. 2018;47(2):299–311. - PubMed
-
- Fleseriu M., Auchus R., Bancos I., Ben-Shlomo A., Bertherat J., Biermasz N.R., Boguszewski C.L., Bronstein M.D., Buchfelder M., Carmichael J.D., Casanueva F.F., Castinetti F., Chanson P., Findling J., Gadelha M., Geer E.B., Giustina A., Grossman A., Gurnell M., Ho K., Ioachimescu A.G., Kaiser U.B., Karavitaki N., Katznelson L., Kelly D.F., Lacroix A., McCormack A., Melmed S., Molitch M., Mortini P., Newell-Price J., Nieman L., Pereira A.M., Petersenn S., Pivonello R., Raff H., Reincke M., Salvatori R., Scaroni C., Shimon I., Stratakis C.A., Swearingen B., Tabarin A., Takahashi Y., Theodoropoulou M., Tsagarakis S., Valassi E., Varlamov E.V., Vila G., Wass J., Webb S.M., Zatelli M.C., Biller B.M.K. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. - PMC - PubMed
-
- Galm B.P., Qiao N., Klibanski A., Biller B.M.K., Tritos N.A. Accuracy of laboratory tests for the diagnosis of cushing syndrome. J. Clin. Endocrinol. Metab. 2020;105(6) - PubMed
-
- Mu D., Fang J., Yu S., Ma Y., Cheng J., Hu Y., Song A., Zhao F., Zhang Q., Qi Z., Zhang K., Xia L., Qiu L., Zhu H., Cheng X. Comparison of direct and extraction immunoassay methods with liquid chromatography-tandem mass spectrometry measurement of urinary free cortisol for the diagnosis of cushing's syndrome. Ann Lab Med. 2024;44(1):29–37. - PMC - PubMed
LinkOut - more resources
Full Text Sources

