Stereoselective chemoenzymatic phytate transformations provide access to diverse inositol phosphate derivatives
- PMID: 40584237
- PMCID: PMC12203238
- DOI: 10.1039/d5sc02844b
Stereoselective chemoenzymatic phytate transformations provide access to diverse inositol phosphate derivatives
Abstract
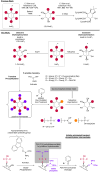
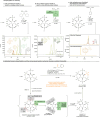
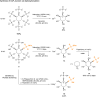
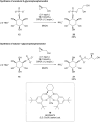
Phosphorylated myo-inositols (InsPs) are essential cytoplasmic signaling molecules, while their lipidated analogs (PtdInsPs) play a crucial role in membrane signaling. Stereoselective synthesis of these compounds has been achieved through various methods, predominantly using the meso compound myo-inositol as a starting material. However, phytate (InsP6), also a meso compound, is the most abundant inositol derivative in plants - far more prevalent than myo-inositol itself. Despite its abundance, phytate has been rarely used in synthetic strategies for accessing a variety of chiral inositol phosphates and their derivatives through selective dephosphorylations on a preparative scale. Here, we report gram-scale (stereo)selective dephosphorylations of phytate using phytases and demonstrate the application of these products in generating modified InsPs through a transient phosphitylation approach. Notably, the bacterial effector XopH efficiently desymmetrizes meso-phytate to yield enantiomerically pure 1-OH-InsP5. This transformation renders the 1-position accessible for further modifications, which, in biological systems, is where glycerolphosphate diesters are attached. By using selective dephosphorylations with phytases in concert with chemoselective telescoping reaction sequences, this approach greatly advances the stereoselective synthesis of inositol phosphates and their derivatives, such as glycerophosphoinositols, from abundant InsP6.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
References
LinkOut - more resources
Full Text Sources

