Genome-Wide Association Study of Intraocular Pressure in Population-Based Cohorts in Japan: The Tohoku Medical Megabank Organization Eye Study
- PMID: 40584246
- PMCID: PMC12206034
- DOI: 10.1016/j.xops.2025.100821
Genome-Wide Association Study of Intraocular Pressure in Population-Based Cohorts in Japan: The Tohoku Medical Megabank Organization Eye Study
Abstract
Purpose: This study was conducted to elucidate the distribution and determinants of ocular biometric parameters and to assess the association between intraocular pressure (IOP) and single nucleotide polymorphisms (SNPs) in the Japanese population-based genome cohort studies.
Design: Cross-sectional analysis involving genome-wide association studies (GWASs).
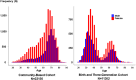
Participants: In total, 22 150 participants aged >18 years from the population cohort (Community-Based Cohort [CommCohort]) and 11 302 participants from the Birth and Three-Generation (BirThree) Cohort of the Tohoku Medical Megabank Organization Eye Study were examined.
Methods: Participant underwent interviews, ophthalmic and physiological examinations, laboratory tests, and microarray analyses. Genome-wide association studies were conducted in the CommCohort (discovery stage) and the BirThree Cohort (replication stage), followed by a meta-analysis. Associations of SNPs and IOP were evaluated using a genome-wide significance threshold (5 × 10- 8).
Main outcome measures: Association of SNPs with IOP and distributions of IOP by sex and age.
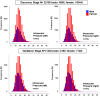
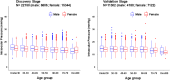
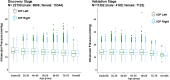
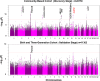
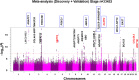
Results: In the discovery stage, the mean IOP of the right and left eye was 13.95 and 14.02 mmHg, respectively. In the replication stage, the corresponding values were 14.32 and 14.27 mmHg, respectively. A significant age-related reduction in IOP was observed in both stages (P < 0.001). Genome-wide association studies identified 573 and 2 genome-wide significant SNPs in the discovery and replication stages, respectively. Meta-analysis revealed 1601 significant SNPs across 21 loci on 11 chromosomes (Chrs). Of these loci, 17 were previously known to be associated with IOP or glaucoma, while four-septin-8 (SEPT8; Chr5), aldehyde dehydrogenase 2 (ALDH2; Chr12), collagen type VI alpha 2 chain (COL6A2; Chr21), and Wnt family member 7B (WNT7B; Chr22)-were newly identified.
Conclusions: This large-scale GWAS in a Japanese population identified 21 loci associated with IOP, including 4 novel loci. The findings highlight both genetic similarities and population-specific variations in SNPs influencing IOP and provide valuable insights to enhance eye health care, including glaucoma management.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Genome-wide association study (GWAS); Intraocular pressure; Population-based cohort study.
© 2025 by the American Academy of Ophthalmologyé.
Figures


Similar articles
-
Genome-wide Association Study of Axial Length in Population-based Cohorts in Japan: The Tohoku Medical Megabank Organization Eye Study.Ophthalmol Sci. 2022 Jan 22;2(1):100113. doi: 10.1016/j.xops.2022.100113. eCollection 2022 Mar. Ophthalmol Sci. 2022. PMID: 36246171 Free PMC article.
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
The Association of Urinary Sodium Excretion with Glaucoma and Related Traits in a Large United Kingdom Population.Ophthalmol Glaucoma. 2024 Sep-Oct;7(5):499-511. doi: 10.1016/j.ogla.2024.04.010. Epub 2024 May 8. Ophthalmol Glaucoma. 2024. PMID: 38723778 Free PMC article.
-
Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty.Cochrane Database Syst Rev. 2017 Feb 23;2(2):CD010746. doi: 10.1002/14651858.CD010746.pub2. Cochrane Database Syst Rev. 2017. PMID: 28231380 Free PMC article.
-
Fornix-based versus limbal-based conjunctival trabeculectomy flaps for glaucoma.Cochrane Database Syst Rev. 2021 Aug 26;8(8):CD009380. doi: 10.1002/14651858.CD009380.pub3. Cochrane Database Syst Rev. 2021. PMID: 34437715 Free PMC article.
References
-
- Jonas J.B., Aung T., Bourne R.R., et al. Glaucoma. Lancet. 2017;390:2183–2193. - PubMed
-
- Heijl A., Leske M.C., Bengtsson B., et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. - PubMed
-
- Leske M.C., Heijl A., Hyman L., et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. - PubMed
-
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

