Visual Outcomes and Safety Profile of "Dropless Vitrectomy" for Epiretinal Membranes
- PMID: 40584249
- PMCID: PMC12205748
- DOI: 10.2147/OPTH.S528711
Visual Outcomes and Safety Profile of "Dropless Vitrectomy" for Epiretinal Membranes
Abstract
Purpose: To assess the clinical effectiveness and safety of Tri-Moxi intravitreal injection in comparison to standard postoperative topical steroid-antibiotic treatment after epiretinal membrane (ERM) removal with pars plana vitrectomy (PPV).
Methods: A retrospective longitudinal cohort study of 278 eyes undergoing ERM removal by PPV was conducted from 2019 to 2023. Group 1 (N = 139) received a triamcinolone acetonide-moxifloxacin (Tri-Moxi) intravitreal injection at the conclusion of surgery, and Group 2 (N = 139) had postoperative standard topical antibiotic-steroid therapy. Clinical changes of best-corrected visual acuity (BCVA), intraocular pressure (IOP), and central foveal thickness (CFT) were evaluated.
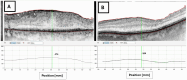
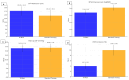
Results: By 3-month after surgery, the Tri-Moxi (Group 1) demonstrated a significantly greater reduction in CFT (178 ± 32 vs 145 ± 38 µm, P < 0.001) and a slightly superior improvement in BCVA (0.34 ± 0.03 vs 0.41 ± 0.04 logMAR, P < 0.05) compared to the standard (Group 2). Postoperative IOP remained minimal change in both groups. The occurrence of cystoid macular edema was markedly reduced in patients receiving Tri-Moxi (4% vs 10%, P = 0.02). No infection or ocular hypertension cases were recorded.
Conclusion: For the postoperative management of PPV in ERM patients, intravitreal Tri-Moxi injection is an efficacious and safe alternative to standard topical therapy. Intravitreal Tri-Moxi can be used as a viable treatment option for managing inflammation and preventing infection after epiretinal membrane removal by pars plana vitrectomy. Tri-Moxi group demonstrated superior anatomical outcomes and comparable functional outcomes while simplifying postoperative care and reducing the occurrence of cystoid macular edema compared to standard topical therapy.
Keywords: central macular edema; drop less; epiretinal membrane; inflammation; moxifloxacin; triamcinolone acetonide; vitrectomy surgery; wound healing.
© 2025 Chalam et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD011599. doi: 10.1002/14651858.CD011599.pub2. Cochrane Database Syst Rev. 2018. PMID: 29669176 Free PMC article.
-
Evaluation of the Efficacy and Safety of Preoperative Intravitreal Triamcinolone Acetonide Combined with Internal Limiting Membrane Peeling for the Treatment of Idiopathic Macular Epiretinal Membrane.Semin Ophthalmol. 2025 Jul;40(5):426-432. doi: 10.1080/08820538.2025.2463999. Epub 2025 Feb 7. Semin Ophthalmol. 2025. PMID: 39921248
-
Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery.Cochrane Database Syst Rev. 2017 Jul 3;7(7):CD010516. doi: 10.1002/14651858.CD010516.pub2. Cochrane Database Syst Rev. 2017. PMID: 28670710 Free PMC article.
-
Ocriplasmin for symptomatic vitreomacular adhesion.Cochrane Database Syst Rev. 2017 Oct 17;10(10):CD011874. doi: 10.1002/14651858.CD011874.pub2. Cochrane Database Syst Rev. 2017. PMID: 29040800 Free PMC article.
-
Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery.Cochrane Database Syst Rev. 2016 Nov 1;11(11):CD006683. doi: 10.1002/14651858.CD006683.pub3. Cochrane Database Syst Rev. 2016. PMID: 27801522 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

