Inclusion of Vitamin E in 1D Nanochannels of 2,4,6-Tris(4-chlorophenoxy)-1,3,5-triazine: Structural and Thermal Characterization of a Stable Solid-State Complex
- PMID: 40584306
- PMCID: PMC12198999
- DOI: 10.1021/acsomega.5c02012
Inclusion of Vitamin E in 1D Nanochannels of 2,4,6-Tris(4-chlorophenoxy)-1,3,5-triazine: Structural and Thermal Characterization of a Stable Solid-State Complex
Abstract
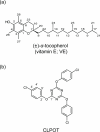
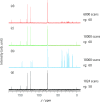
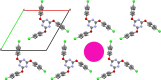
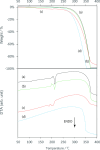
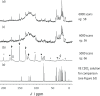
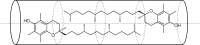
Vitamin E (VE; (±)-α-tocopherol) is a potent antioxidant that plays a critical role in protecting cells from oxidative stress. However, its effectiveness is hindered by its susceptibility to oxidation during storage and poor absorption in the digestive system. Conventional stabilization methods often rely on chemical modifications or the excessive use of excipients, which can reduce VE's effectiveness. Therefore, a delivery system capable of preserving the antioxidant properties of VE without altering its chemical structure is essential. In this study, we develop an inclusion compound in which VE is encapsulated within the 1D nanochannels of 2,4,6-tris-(4-chlorophenoxy)-1,3,5-triazine (CLPOT), forming [(CLPOT)2-(VE)0.49]. These nanochannels physically isolate VE from environmental oxidizing agents to prevent oxidation. In addition, structural and thermal analyses, including powder and single-crystal X-ray diffraction, thermogravimetry-differential thermal analysis, and solution (1H or 13C) and solid-state (13C) nuclear magnetic resonance, confirm stable incorporation of VE into the CLPOT nanochannels consisting of periodical hexagonal CLPOT crystals without requiring chemical modification. Compared with conventional encapsulation techniques, this method provides enhanced protection by creating a confined and protective environment. These findings suggest that CLPOT-based systems offer a promising platform for stabilizing and delivering fat-soluble nutrients, such as VE.
© 2025 The Authors. Published by American Chemical Society.
Figures



Similar articles
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Technological aids for the rehabilitation of memory and executive functioning in children and adolescents with acquired brain injury.Cochrane Database Syst Rev. 2016 Jul 1;7(7):CD011020. doi: 10.1002/14651858.CD011020.pub2. Cochrane Database Syst Rev. 2016. PMID: 27364851 Free PMC article.
-
Interventions to prevent occupational noise-induced hearing loss.Cochrane Database Syst Rev. 2017 Jul 7;7(7):CD006396. doi: 10.1002/14651858.CD006396.pub4. Cochrane Database Syst Rev. 2017. PMID: 28685503 Free PMC article.
References
-
- Gangurde A. B., Ali M. T., Pawar J. N., Amin P. D.. Encapsulation of vitamin E acetate to convert oil to powder microcapsule using different starch derivatives. J. Pharm. Invest. 2017;47:559–574. doi: 10.1007/s40005-016-0287-3. - DOI
-
- Gawrysiak-Witulska M., Siger A., Nogala-Kalucka M.. Degradation of tocopherols during near-ambient rapeseed drying. J. Food Lipids. 2009;45:209–221. doi: 10.1111/j.1745-4522.2009.01164.x. - DOI
-
- Desmarchelier C., Tourniaire F., Prévéraud D. P., Samson-Kremser C., Crenon I., Rosilio V., Borel P.. The distribution and relative hydrolysis of tocopheryl acetate in the different matrices coexisting in the lumen of the small intestine during digestion could explain its low bioavailability. Mol. Nutr. Food Res. 2013;57:1237–1245. doi: 10.1002/mnfr.201200720. - DOI - PubMed
LinkOut - more resources
Full Text Sources
