Establishing data governance for sharing and access to real-world data: a case study
- PMID: 40584737
- PMCID: PMC12206003
- DOI: 10.1093/jamiaopen/ooaf041
Establishing data governance for sharing and access to real-world data: a case study
Abstract
Importance: Data governance, the policies, and procedures for managing data, is a critical factor for secondary use of clinical data for research.
Objectives: This paper describes the evolution of an academic health-care organization's data governance for research, development of an external data sharing process, implementation of related processes, continuous improvement, and ongoing observations of data governance maturity.
Materials and methods: The program was designed to improve the access to and sharing of real-world data for research. Using a combination of qualitative and quantitative methods, we evaluated the program's effectiveness.
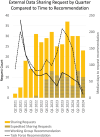
Results: Our results describe a significant improvement in data accessibility as seen in new data-driven performance indicators and in data understanding indicated by new processes, policies, and strategies.
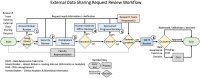
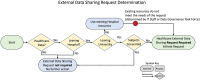
Discussion: The paper outlines the development of a data governance process at an academic health center to support external data sharing, emphasizing the importance of data literacy, cross-office collaboration, and structured workflows to manage complex review requirements. The formalized process improved data access, identified gaps, and enabled continuous quality improvement, though it introduced new bottlenecks and required navigating multi-office reviews and researcher education.
Conclusion: These findings suggest data governance practices that may apply to other institutions.
Keywords: Enterprise Data Warehouse for Research; clinical research data; data governance; external data sharing for research; real-world data; research data.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
Accreditation through the eyes of nurse managers: an infinite staircase or a phenomenon that evaporates like water.J Health Organ Manag. 2025 Jun 30. doi: 10.1108/JHOM-01-2025-0029. Online ahead of print. J Health Organ Manag. 2025. PMID: 40574247
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Strategies for enhancing the implementation of school-based policies or practices targeting risk factors for chronic disease.Cochrane Database Syst Rev. 2017 Nov 29;11(11):CD011677. doi: 10.1002/14651858.CD011677.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Aug 29;8:CD011677. doi: 10.1002/14651858.CD011677.pub3. PMID: 29185627 Free PMC article. Updated.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Governance arrangements for health systems in low-income countries: an overview of systematic reviews.Cochrane Database Syst Rev. 2017 Sep 12;9(9):CD011085. doi: 10.1002/14651858.CD011085.pub2. Cochrane Database Syst Rev. 2017. PMID: 28895125 Free PMC article.
References
-
- Khatri V, Brown CV. Designing data governance. Commun ACM. 2010;53:148-152.
-
- Tse D, Chow, C-k. Ly, T-p, Tong C-y, Tam K-w. The challenges of big data governance in healthcare. In: 17th IEEE International Conference on Trust, Security and Privacy in Computing and Communications (IEEE Trustcom)/12th IEEE International Conference on Big Data Science and Engineering (IEEE Bigdatase). IEEE; 2018:1632-1636.
-
- Fraser P, Moultrie J, Gregory M. The use of maturity models/grids as a tool in assessing product development capability. In: IEEE International Engineering Management Conference, Cambridge, UK, Vol. 1. IEEE; 2002:244-249. 10.1109/IEMC.2002.1038431 - DOI
Publication types
LinkOut - more resources
Full Text Sources
