Chimeric Antigen Receptor T-cell therapy in systemic autoimmune rheumatic diseases: current insights and future prospects
- PMID: 40584766
- PMCID: PMC12202281
- DOI: 10.4078/jrd.2024.0122
Chimeric Antigen Receptor T-cell therapy in systemic autoimmune rheumatic diseases: current insights and future prospects
Abstract
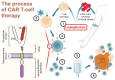
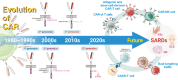
Chimeric Antigen Receptor (CAR) T-cell therapy, revolutionary in treating hematological malignancies, is emerging as a promising approach for systemic autoimmune rheumatic diseases (SARDs). This review examines the potential of CAR T-cell therapy in treating conditions such as systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and idiopathic inflammatory myopathies (IIMs). The evolution of CAR T cells technology, from first to fifth generation, has enhanced its efficacy and persistence. Early clinical studies in SARDs have shown encouraging results, with some patients achieving drug-free remission. CD19-targeted CAR T cells have demonstrated significant B-cell depletion and clinical improvement in patients with SLE, SSc, and IIMs. Despite promising outcomes, challenges remain, including cytokine release syndrome and the need for careful patient selection. Future directions include exploring dual-targeting CARs, chimeric autoantibody receptors (CAARs), and alternative cell sources like γδ T cells, regulatory T cells, natural killer cells. The integration of CAR-based cell therapy into treatment paradigms of patients with SARDs requires further research to optimize efficacy, mitigate side effects, and identify suitable target biomarkers. While hurdles exist CAR-based cell therapy holds the potential to revolutionize management of patients with SARDs, offering hope for long-term, drug-free remission in these complex autoimmune conditions.
Keywords: Autoimmune diseases; Chimeric Antigen Receptor T-cell therapy; Idiopathic inflammatory myopathy; Systemic lupus erythematosus; Systemic sclerosis.
Copyright © 2025 by The Korean College of Rheumatology. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Chimeric antigen receptor cell therapy: A revolutionary approach transforming cancer treatment to autoimmune disease therapy.Autoimmun Rev. 2025 Aug 29;24(9):103859. doi: 10.1016/j.autrev.2025.103859. Epub 2025 Jun 23. Autoimmun Rev. 2025. PMID: 40562292 Review.
-
[CAR T cells in non-malignant diseases].Inn Med (Heidelb). 2025 Aug;66(8):810-817. doi: 10.1007/s00108-025-01945-x. Epub 2025 Jun 30. Inn Med (Heidelb). 2025. PMID: 40586812 Review. German.
-
Revaccination following CAR-T therapy: a needs assessment.Hematology. 2025 Dec;30(1):2519865. doi: 10.1080/16078454.2025.2519865. Epub 2025 Jun 23. Hematology. 2025. PMID: 40550220
-
Transduction of γδ T cells with Baboon envelope pseudotyped lentiviral vector encoding chimeric antigen receptors for translational and clinical applications.Front Immunol. 2025 Jun 6;16:1548630. doi: 10.3389/fimmu.2025.1548630. eCollection 2025. Front Immunol. 2025. PMID: 40547029 Free PMC article.
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
