Developing the Strategy to Use Silk Spheres for Efficient, Targeted Delivery of Oligonucleotide Therapeutics to Cancer Cells
- PMID: 40584785
- PMCID: PMC12204100
- DOI: 10.2147/IJN.S519906
Developing the Strategy to Use Silk Spheres for Efficient, Targeted Delivery of Oligonucleotide Therapeutics to Cancer Cells
Abstract
Introduction: Oligonucleotide-based drugs, such as siRNA, hold great promise for disease treatment, including cancer. However, their clinical application has challenges related to cell-specific delivery and susceptibility to degradation. The use of drug delivery systems (DDS) may address these problems. Nanoparticles of bioengineered spider silk demonstrate significant potential as DDS due to their biocompatibility and biodegradability. Another advantage of this material is the possibility of functionalization, which allows the control of its property. The main objective of this study was to develop a strategy for targeted delivery of oligonucleotide-based therapeutics into cancer cells using bioengineered silk technology.
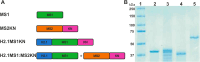
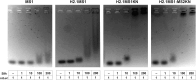
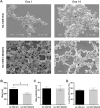
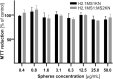
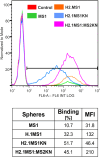
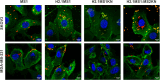
Materials and methods: Two spider silk spheres that bind oligonucleotides and target cancer cells that overexpress HER2 (HER2+) were constructed. One type of sphere was made of a newly designed silk, H2.1MS1KN, which contained two functional peptides: H2.1 for binding HER2 and KN for binding oligonucleotide. The second type of sphere was formed of a blend of two previously described proteins, H2.1MS1 and MS2KN; these proteins differed not only in the functional domain (H2.1 vs KN) but also in the sequence of silk (MS1 vs MS2). The ability of proteins to bind oligonucleotides was analyzed via gel electrophoresis. The biophysicochemical properties of particles were analyzed using an SEM, NanoSight, ZetaSizer, flow cytometry, and scanning confocal microscopy. The silk particle potential was analyzed using siRNA for silencing STAT3 expression in the HER2+ breast cancer model.
Results: Both H2.1MS1KN and H2.1MS1:MS2KN proteins efficiently bound nucleic acid. H2.1MS1:MS2KN formed smaller spheres than H2.1MS1KN. Although both H2.1MS1KN and blended H2.1MS1:MS2KN spheres were effectively loaded with oligonucleotides, only H2.1MS1:MS2KN spheres delivered siRNA to HER2+ cancer cells that successfully silenced STAT3 expression.
Conclusion: Not only the selection of functional peptides but also their quantity and type of silk is crucial when developing an effective silk-based DDS for delivering active siRNA.
Keywords: STAT3; bioengineered spider silk; cancer therapy; siRNA-delivery; spheres; targeted delivery.
© 2025 Molenda et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
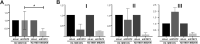
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

