Mitochondrial metabolic regulation of macrophage polarization in osteomyelitis and other orthopedic disorders: mechanisms and therapeutic opportunities
- PMID: 40584964
- PMCID: PMC12202483
- DOI: 10.3389/fcell.2025.1604320
Mitochondrial metabolic regulation of macrophage polarization in osteomyelitis and other orthopedic disorders: mechanisms and therapeutic opportunities
Abstract
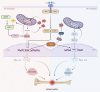
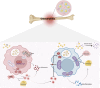
Osteomyelitis is a complex infectious bone disease involving pathogen invasion, host immune responses, and dysregulation of the local microenvironment. As a critical component of the innate immune system, macrophages play a pivotal role in inflammatory responses and tissue repair. Their polarization states (M1/M2) directly influence disease progression, while mitochondrial metabolism, as the central hub of cellular energy metabolism, has recently been shown to play a key role in macrophage polarization and functional regulation. However, how mitochondrial metabolism regulates macrophage polarization to affect the pathological mechanisms of osteomyelitis, and how to develop novel therapeutic strategies based on this mechanism, remain critical scientific questions to be addressed. This review systematically summarizes the molecular mechanisms by which mitochondrial metabolism regulates macrophage polarization and its role in osteomyelitis, with a focus on the impact of mitochondrial dynamics (fission/fusion), metabolic reprogramming, and reactive oxygen species (ROS) generation on macrophage polarization. Additionally, potential therapeutic strategies targeting mitochondrial metabolism are analyzed. For the first time, this review integrates the interplay between mitochondrial metabolism and macrophage polarization in osteomyelitis, revealing how mitochondrial dysfunction exacerbates inflammation and bone destruction through metabolic reprogramming. Based on these findings, we propose novel therapeutic strategies targeting mitochondrial metabolism, offering new perspectives and directions for understanding the pathogenesis and clinical treatment of osteomyelitis.
Keywords: bone repair; inflammation; macrophage polarization; mitochondrial dynamics; mitochondrial metabolism; osteomyelitis.
Copyright © 2025 Li, Zhang, Peng, Zhao, Li, Yu, Huang, Yang, Deng, Yang, Zhang and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Reactive Oxygen Species in Asthma: Regulators of Macrophage Polarization and Therapeutic Implications: A Narrative Review.J Asthma Allergy. 2025 Jul 25;18:1129-1146. doi: 10.2147/JAA.S529371. eCollection 2025. J Asthma Allergy. 2025. PMID: 40735422 Free PMC article. Review.
-
Immunometabolism of macrophages in the bone microenvironment: a new perspective for bone healing therapy.J Adv Res. 2025 Jul 29:S2090-1232(25)00576-4. doi: 10.1016/j.jare.2025.07.046. Online ahead of print. J Adv Res. 2025. PMID: 40744273 Review.
-
Regulation of macrophage plasticity by circCCDC719-13 through HSP90 inhibition suppresses prostate cancer progression and metastasis: a translational study.Int J Surg. 2025 Jun 27. doi: 10.1097/JS9.0000000000002895. Online ahead of print. Int J Surg. 2025. PMID: 40576192
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Intracellular survival of Staphylococcus aureus in macrophages during osteomyelitis.Virulence. 2025 Dec;16(1):2553789. doi: 10.1080/21505594.2025.2553789. Epub 2025 Sep 1. Virulence. 2025. PMID: 40888369 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

